Case Studies: Drug Interactions and Adverse Drug Reactions
EMR alerts can be life-saving, but they can also lead to alert fatigue and errors. We walk through four cases that detail how important these alerts can be!

The article is in electronic format on your phone screen. You continue to get alerts and notifications the entire hour you are reading. Then, seven days later, I ask you to give a detailed presentation on what you read. Oh, and you do not get to reference the article again.
- Would you be able to do it?
- How many details would you be able to recall?
Unless you are blessed with a photographic memory, you would (probably) only be able to present about big-picture information and maybe a few stats you found interesting. Especially with all the alerts from text messages, Facebook, e-mail, etc., your attention and focus would have been diverted the entire hour you read the article. How are you supposed to remember any information for a detailed presentation 7 days later?
Unfortunately, this is similar to real-life clinical practice. You are expected to take information crammed into your brain from school, remember specific details, apply this information constantly throughout the day, and manage all the pop-ups, phone calls, interaction alerts, and warnings at the same time. You have to do all of this while keeping pristine focus to avoid errors.
This is a recipe for alert fatigue, prescribing errors, and missed mistakes on all members of the healthcare team. We are all double-checks for each other. To drive the point home (and maybe provide some interesting clinical scenarios), let's dive into a few cases about Clinical Pharmacy Medication Safety!
A big thank you to Amanda Ingemi, Pharm.D., MPH for sharing her slides from a lecture with Samuel Ikonne, Ph.D., and Bill Wightkin, Pharm.D., M.S. This lecture was presented in the Clinical Pharmacy and Medication Safety Elective at our local medical school.

Case 1 - Anti-Fungals
You have a patient on amphotericin and they are having side effects.
You are rounding with the infectious disease service, and you agree on rounds to change the patient to posaconazole.
You dismiss these alerts...
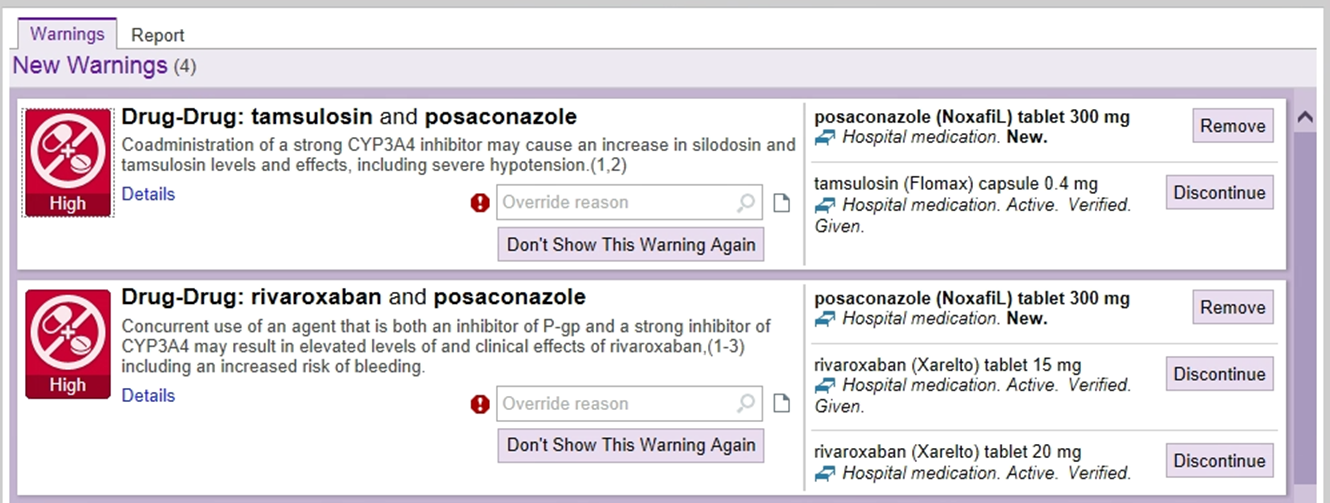
The pharmacist calls you regarding these same alerts.
- What do you do?
- What are your options?
Drug Interaction Options
- Stop one of the agents.
- Change the new drug (posaconazole in this case) to something that does NOT have the interaction.
- Replace the current drug (rivaroxaban, tamsulosin) to something that doesn’t have the drug interaction.
- Manage the drug interaction.
- Decrease/increase the dose of the drug effected (i.e. decrease rivaroxaban, change tamsulosin to every other day dosing – you cannot obtain a smaller dose than 0.4 mg capsules).
- Watch for side effects of the increased or decreased drug.

Case 2 - Statins
You are in an outpatient clinic following a patient for her hypertension, hyperlipidemia and diabetes. Their heart rate has been high (90s) and blood pressure over the last couple visits have been in the 160s/90s.
They are currently taking:
- Simvastatin 40 mg every day
- Lisinopril 40 mg every day
- Metformin 1,000 mg twice daily
You plan to start diltiazem 360 mg and get a pop-up warning about an interaction between diltiazem and simvastatin concomitant therapy.
Questions:
- How would you investigate this drug-drug interaction?
- You could expand the alert pop-up for an explanation in your health system's EMR, or you could go to Lexi-comp/Micromedex and use the Drug Interaction Checker (screenshots below).

- Is the interaction severe enough that you would stop the drug or just monitor?
- This will vary by pharmacist and provider. Welcome to the world of medicine! Nothing is black and white.
- The best answer here would either be to lower the dose of simvastatin to 10 mg daily or to switch to an alternative HMG-CoA-reductase inhibitor (statin) that does not interact with diltiazem (i.e. rosuvastatin, pravastatin).
- If monitoring, what are you monitoring for? If stopping the drug, what alternative medication would you consider adding?
- Lexi-comp helped us with this question. We would monitor for muscle pain (myopathy) and dark urine/elevated CPK/LFTs (rhabdomyolysis). We are primarily worried about simvastatin toxicity in this interaction.
- However, simvastatin can decrease diltiazem levels as well, and we should be monitoring vital signs to ensure the new drug, diltiazem, and controlling the patient's HR and BP (which is why we started the medication).
- An alternative medication we could consider is a different statin (mentioned above) or a beta-blocker such a carvedilol to help lower both HR and BP without interacting with simvastatin.
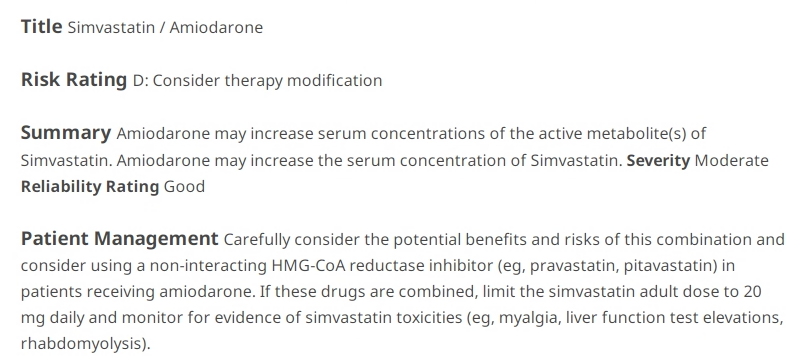
- If the added drug was amiodarone, would this change your approach?
- The only aspect that differs between the amiodarone-simvastatin interaction when compared to the diltiazem-simvastatin interaction is the max recommended simvastatin dose of 20 mg with concomitant use of amiodarone. However, we would still need to monitor for the same toxicities of increased simvastatin levels (due to metabolism inhibition from amiodarone).
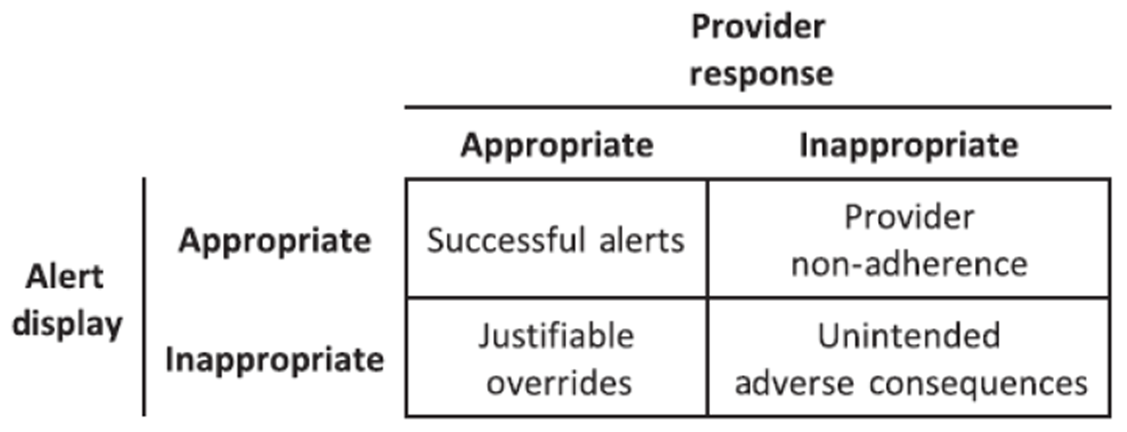
Alert Fatigue
What is "alert fatigue?" Throughout the day, providers (and the nurses/pharmacists/etc.) receive a lot of pop-ups and alerts when entering orders. The alerts are set-up to provide quality safety information and warnings. If the alerts become spam-like, inappropriate, or repetitive, the health care team member(s) could start to inappropriately override or by-pass the alerts. This could lead to unintended consequences and patient harm. See the graphic below from McCoy et al. about this concept.
I decided to bring up the concept of alert fatigue to help remind you how important alerts are in the EMR. If you feel strongly that an alert is inappropriate, there is usually a comment box that will allow you to send feedback directly to IT. Maybe there are sufficient individuals providing the same feedback, and eventually the alert can be removed if deemed clinically appropriate. I also want to remind all of the pharmacy students, residents, and pharmacists out there that our job is to address every alert and contact the provider with recommended management of drug interactions. Yes, the providers have responsibility as well, but we are that vital second check! Don't be a victim to alert fatigue.

Case 3 - The Consequences of Alert Fatigue
On the Infectious Disease service, the team consults on a patient who is diagnosed with esophagitis caused by a Candida albicans infection. The patient is a 48-year-old male who has T2DM, hypertension, and an HIV infection.
Current medications are metformin, lisinopril, chlorthalidone and antivirals (efavirenz, emtricitabine, and tenofovir). For the esophagitis, the resident places an order into the EMR for voriconazole 200 mg orally 2 times day.
Within the next hour, the resident is contacted by a pharmacist wondering if they saw the drug interaction alert involving voriconazole. The resident has no recollection of seeing this alert. Upon investigation, the alert was displayed upon order entry, but needed action was not taken, and the provider had selected ‘benefit outweighs risk.’
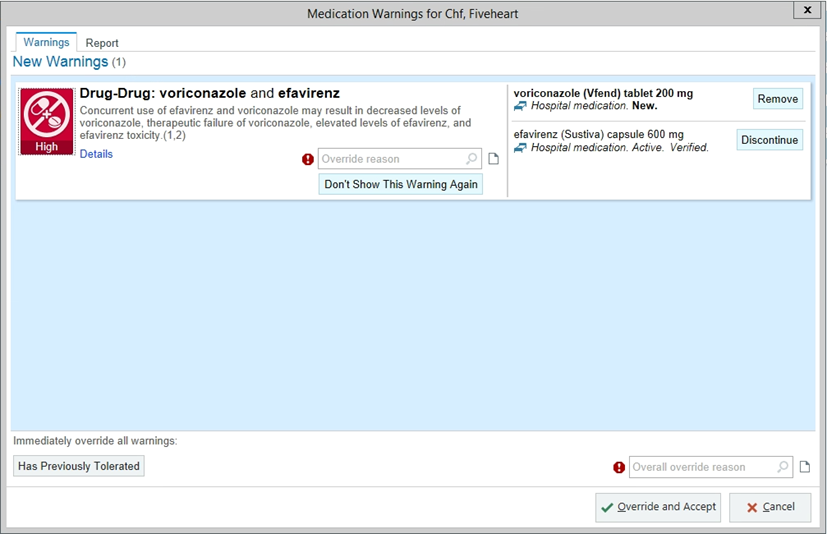
Questions:
- What are some reasons why the resident missed the drug interaction alert?
- Alert fatigue! HIV medications can populate a lot of drug interaction alerts. This resident may also be thinking "this esophagitis therapy is only for 14 days, the patient will be fine." Or, the resident may be fatigued themselves and/or relying on pharmacy to be their double check.
- How can these alert dismissals be avoided in the future?
- Slow down! Easier said than done, of course.
- What should have been the correct response to the drug interaction alert?
- For Candida albicans esophagitis, the better choice would be fluconazole. Although in the same drug class, there are some differences between fluconazole and voriconazole, metabolism-wise. I have included 5-snippets below that you can click on to enlarge and review.





- Due to the differences in CYP substrates/inhibitors of the three medications, the combination of fluconazole/efavirenz is a B-risk rating for QTC-prolonging concerns. This should prompt a review of heart-conditions on the patient's profile in addition to the potential of CYP3A4 interaction management. Fluconazole is a moderate CYP3A4 inhibitor (voriconazole is a major inhibitor) and efavirenz is a substrate for this enzyme. Although Lexi-comp does not provide specifics on this, a discussion should be had between the primary team and pharmacy on whether a reduced dose of efavirenz (300 mg daily) should be initiated while on fluconazole therapy.
- Another approach to this situation would be to stick with voriconazole and efavirenz. Lexi-comp actually provides a detailed recommendation that I will copy below:
- The voriconazole prescribing information states that concurrent use of standard voriconazole doses with efavirenz doses of 400 mg/day or higher is contraindicated. If these drugs are to be coadministered, the voriconazole oral maintenance dose should be increased to 400 mg every 12 hours, and the efavirenz dose should be reduced to 300 mg daily. - Lexi-comp 2023
Increase metabolism (inducer)
• Rifampin
• Phenytoin
• Phenobarbital
• Carbamazepine
Decrease metabolism (inhibitor)
• Ritonavir/Cobicistat
• Azole antifungals (posaconazole, voriconazole)
• Grapefruit
• Amiodarone
Transporter inhibitors
Medications with similar side effects
• Sometimes considered a drug-drug interaction since there’s a synergistic adverse effect

Case Four - Immunosuppressants
A transplant patient is admitted with a tacrolimus overdose attempted suicide. The tacrolimus level is >60 ng/ml (normal goal 4-10 ng/mL). One side effect of tacrolimus is lowering the seizure threshold.
Questions:
- What medication could you start for a drug-drug interaction that would be helpful in preventing seizures?
- Phenytoin! Not only is phenytoin an anti-seizure medication, phenytoin is a known CYP-inducer (specifically CYP3A4). It just so happens that tacrolimus is metabolized by CYP3A4. By starting phenytoin, we can protect against the patient's lower-seizure threshold caused by elevated levels of tacrolimus AND we can help increase the metabolism of tacrolimus to lower the concentration faster.
- More on phenytoin for tacrolimus toxicity
- What other medications might be affected by this drug-drug interaction?
- Other medications that are metabolized by any of the CYP enzymes/efflux transporters that phenytoin induces: CYP1A2 (weak), CYP2B6 (weak), CYP3A4 (strong), P-glycoprotein/ABCB1, UGT1A1, UGT1A4. Any affected medications may require a higher dose while on phenytoin therapy.
- How long will this interaction take to wear off?
- To induce CYP enzymes, that means the production of the enzyme is ramped up. Therefore, there will be a short delay until the increase of enzyme is complete and active. The same is true upon discontinuing the enzyme inducer. It can take a few days up to 2-3 weeks after discontinuing the induction medication to return CYP levels back to normal. The half-life of the medication is the determining factor and can vary between different CYP inducers.
- Brief Pharmacy Times article about "Time Course for Enzyme Induction and Deinduction"
Discussion on Adverse Drug Reactions
What differentiates an Adverse Drug Reaction (ADR) and a Medication Error?
As shown in the graphic below, an ADR is something that is not preventable and is due to an inherent risk to the drug itself and a patient specific factor. A medication error is when there are is a preventable adverse drug event that occurs. This can happen when the risk of a medication is known upon prescribing but the alert is bypassed, a medication is administered incorrectly, renal adjustments were not made, labs were not monitored, etc.
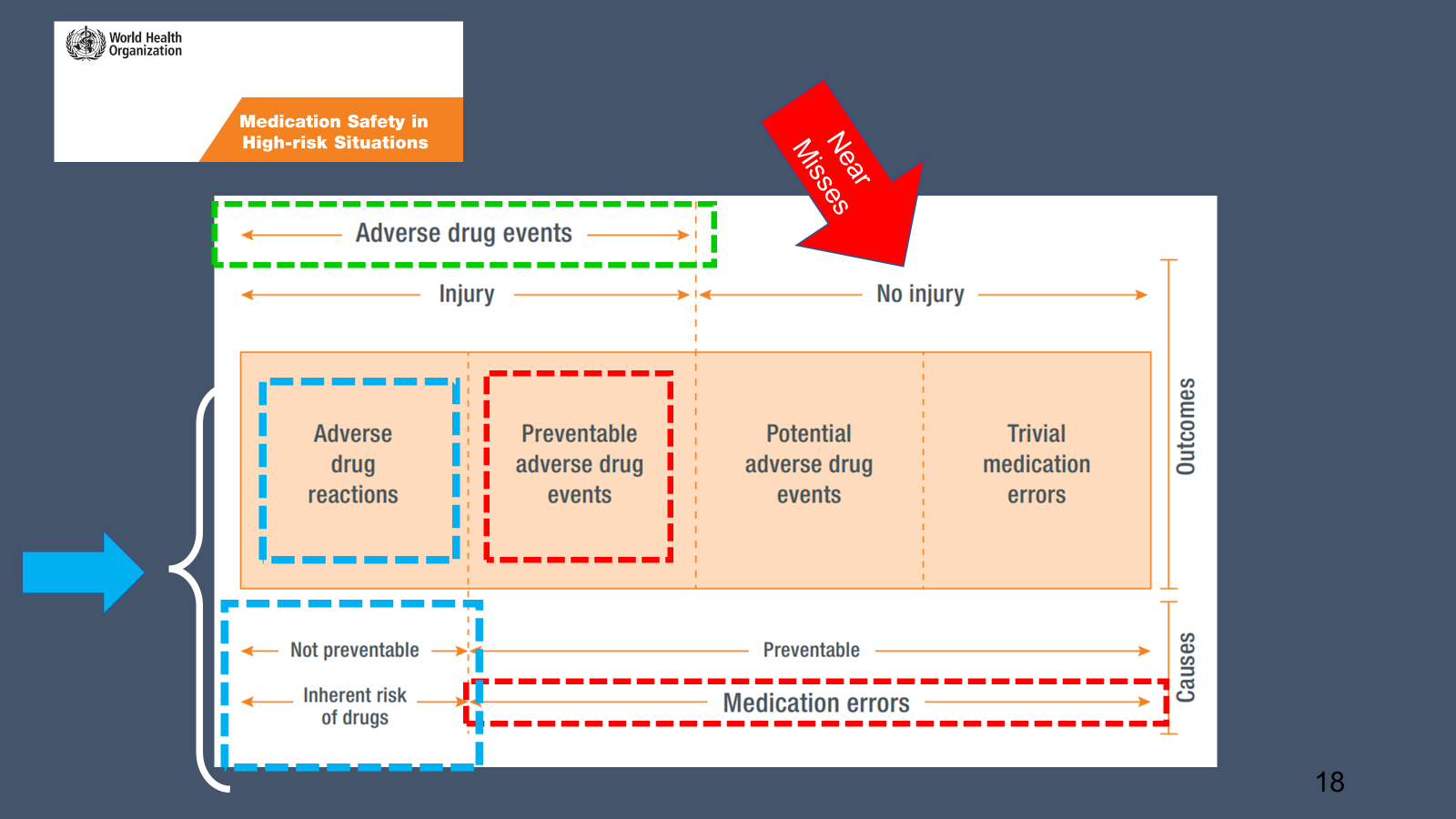
If you find yourself in the care of a patient that experiences a true ADR (the situation outlined in light blue boxes in the above graphic), it is your responsibility to report this to the FDA MedWatch program (see below).

Adverse Effect? Add it to Patient Allergies!
Now, if you find yourself in the care of a patient that does have an allergic reaction to a specific medication (i.e. ampicillin) or has a positive HITT panel (precludes the patient from receiving heparin for anticoagulation) --> ADD IT TO THE ALLERGY LIST! This will ensure the patient stays safe in the future!
What I find myself frequently doing is "correcting" old penicillin allergies in the EMR. Many times, a patient can a documented allergy to pencillins "as a child" and they "cannot remember the reaction." I think can search in Epic for related antibiotics such as aminopenicillins, cephalosporins, etc. If the patient has received cefazolin post-op in the past, I can go into the penicillin allergy and add more detail by writing when and what medication they patient has tolerated in the past. This will help future providers and pharmacists made safe and appropriate care decisions with the most information possible.
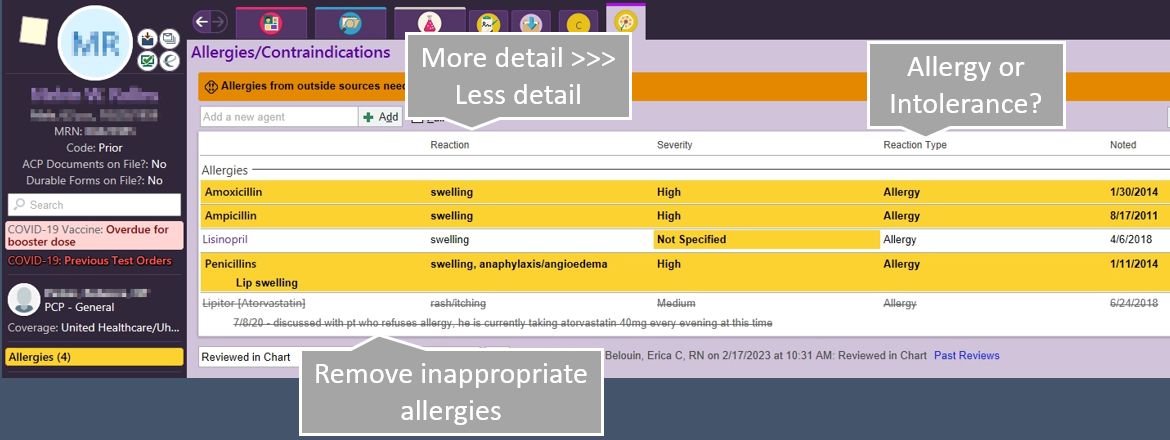
Side Effects vs. Allergy
If you are going to document in the allergy section of the patient's chart, but sure to differentiate between an intolerance, side effect, and true allergy to a medication.
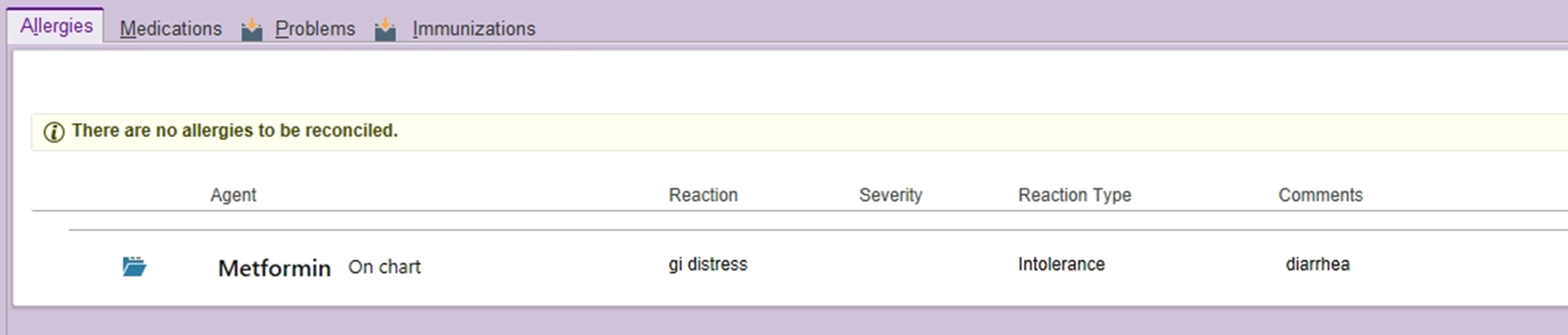
An intolerance might be "gi distress" to augmentin or the metformin example shown below. This doesn't preclude the patient from receiving all penicillins, though. The patient had some stomach upset with the oral formulation, and may tolerate the IV or suspension formulations of augmentin better (IV = unasyn; suspension = augmentin).
An example for an intolerance or side effect would be muscle pains (myalgias) to a specific high intensity statin. This is not a true allergy, but a side effect that we may be able to re-challenge with a lower dose of a different statin.
A true allergy, such as hives, anaphylaxis, skin rash, etc. should also be document with as much detail as you deem appropriate.
Concluding Thoughts
Did the cases make sense? Did they get your mind turning? Maybe they showed you how important all the little interactions and metabolism pathways can be in practice!
Big takeaways
- Don't fall victim to alert fatigue
- Take your time
- Have a questioning attitude
- Use your resources
- If you see something, say something!
- Document, document, document
- Be a good wingman/wingwoman and add allergies/details to the chart
- Patient safety is always our top priority!
*Information presented on RxTeach does not represent the opinion of any specific company, organization, or team other than the authors themselves. No patient-provider relationship is created.

