Chronic Graft-Versus-Host Disease (cGVHD)
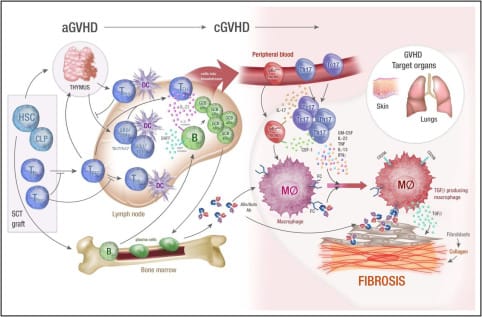
cGVHD differs fundamentally from acute GVHD and resembles features of autoimmunity and chronic inflammation-driven fibrosis. The pathogenesis includes three overlapping phases.
Overview:
Chronic graft-versus-host disease (cGVHD) is a multi-organ, immune-mediated, fibrotic disease that arises in 30–70% of patients who survive beyond 100 days following allogeneic hematopoietic stem cell transplantation (allo-HSCT). It represents the leading cause of late non-relapse mortality and significantly reduces patient quality of life.
Pathophysiology:
cGVHD differs fundamentally from acute GVHD and resembles features of autoimmunity and chronic inflammation-driven fibrosis. The pathogenesis includes three overlapping phases:
- Early Inflammation
- Initiated by tissue injury from conditioning regimens and alloimmune T-cell responses.
- Proinflammatory cytokines (IL-6, TNF-α, IL-1) and DAMPs activate APCs and prime donor T-cells.
- Immune Dysregulation
- Breakdown of central and peripheral tolerance.
- Expansion of Th17 cells, T follicular helper cells, and pathogenic B-cell subsets.
- Impaired regulatory T-cell (Treg) homeostasis.
- Elevated BAFF levels drive B-cell survival and autoantibody production.
- Fibrosis and End-Organ Damage
- Persistent inflammation leads to fibroblast activation, myofibroblast differentiation, and excessive extracellular matrix deposition.
- Fibrosis contributes to bronchiolitis obliterans syndrome (BOS), scleroderma-like skin, and joint contractures.
Immune Cell Players: Th17, CD4+ Th, Tregs, autoreactive B cells, macrophages, fibroblasts
Key Mediators: TGF-β, IL-17, IL-21, IFN-γ, BAFF, PDGF
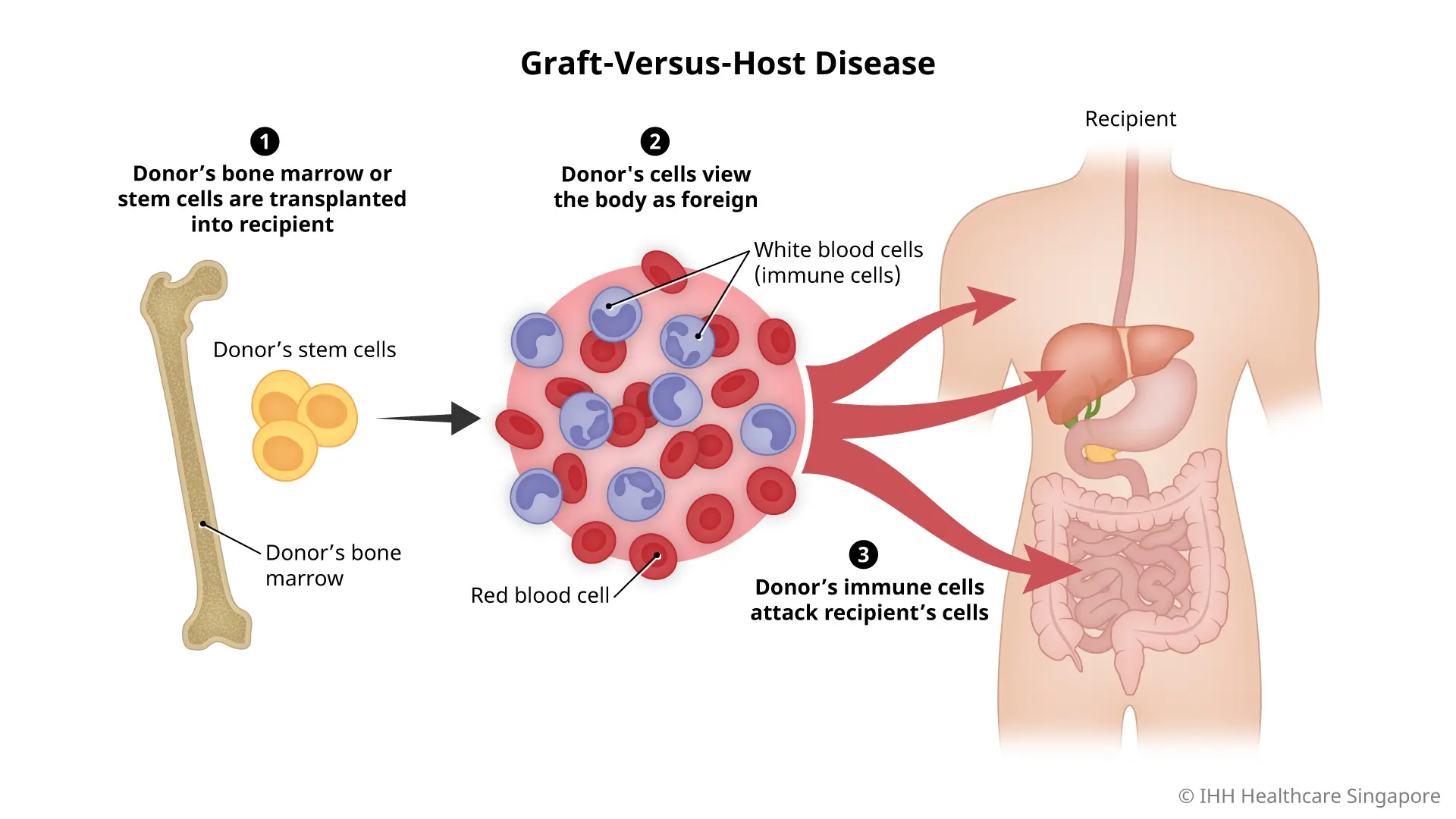
Risk Factors:
- Prior acute GVHD (especially Grade ≥ II)
- Peripheral blood stem cell (PBSC) grafts (vs bone marrow)
- HLA mismatch or unrelated donor
- Older age, especially donor > recipient
- Female donor to male recipient
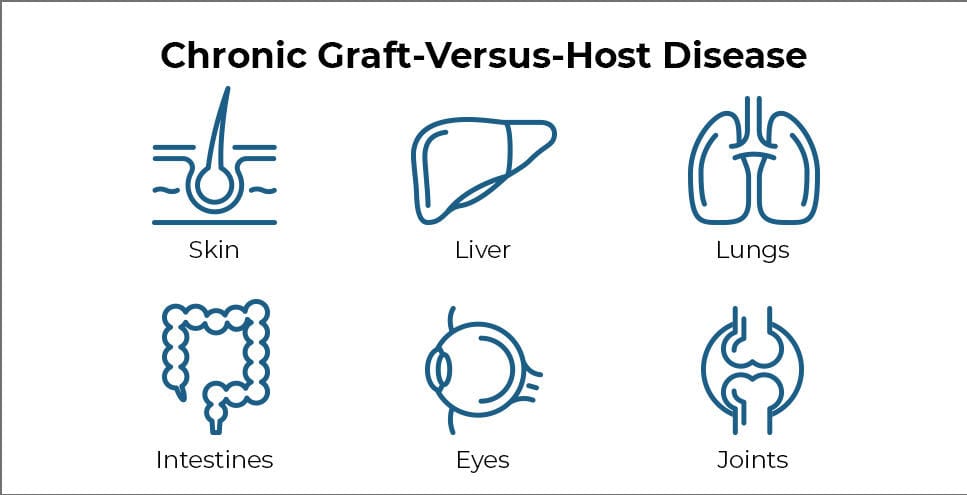
Clinical Manifestations:
Diagnosis and grading follow the NIH 2014 consensus criteria. Disease is classified as mild, moderate, or severe based on organ involvement and functional impact.
Current Treatment Landscape:
First-Line Therapy:
- Systemic corticosteroids
- Response rate ~50%, but can result in steroid-related toxicity long-term
FDA-Approved Agents for Steroid-Refractory or Steroid-Dependent cGVHD:
Other Investigational/Off-Label Agents: not all-inclusive
Prognosis and Outcomes:
- Severe cGVHD: Reduced functional status, high infectious complications, long-term immunosuppression
- Mortality: Non-relapse mortality (NRM) up to 30–40% in refractory cases
- Ongoing need for biomarker-driven treatment, fibrosis-specific therapy, and steroid-sparing regimens

Conclusion:
Chronic GVHD remains a significant barrier to long-term survival and quality of life in patients undergoing allogeneic hematopoietic stem cell transplantation. It is a heterogeneous disease with overlapping autoimmune, alloimmune, and fibrotic mechanisms that manifest across multiple organ systems. Historically managed with prolonged corticosteroids and broad immunosuppressants, cGVHD therapy has advanced substantially in recent years with the approval of targeted agents such as ibrutinib, ruxolitinib, belumosudil, and most recently axatilimab.
Each approved therapy addresses a distinct immunopathogenic pathway: B-cell signaling (ibrutinib), cytokine-driven inflammation (ruxolitinib and belumosudil), and fibrotic macrophage activity (axatilimab). This highlights the shift toward mechanism-based, organ-specific, and steroid-sparing strategies.
Next Steps:
Moving forward, the field is poised to benefit from biomarker-driven treatment selection, earlier intervention, and combination approaches tailored to the dominant biological process in each patient—whether inflammatory, autoimmune, or fibrotic. Continued translational research and clinical trial integration will be essential to refine these strategies and improve outcomes for patients with this challenging and multifaceted disease.
Key References:
- Zeiser R, Blazar BR. Mechanisms of Chronic Graft-versus-Host Disease. N Engl J Med. 2017;377(26):2565–79.
- Jagasia M, et al. Ruxolitinib vs Best Available Therapy in cGVHD: REACH3. Lancet. 2021;398(10308):1682–1691.
- Miklos DB, et al. Ibrutinib in chronic GVHD. Blood. 2017;130(21):2243–2250.
- Chen Y-B, et al. Belumosudil for cGVHD after ≥2 prior lines: ROCKstar study. J Clin Oncol. 2021;39(17):1888–1898.
- Jaglowski SM, et al. AGAVE-201: Axatilimab in patients with chronic GVHD. ASH 2023, Abstract #LBA3.
*Information presented on RxTeach does not represent the opinion of any specific company, organization, or team other than the authors themselves. No patient-provider relationship is created.

