DELIVER Trial
The New England Journal of Medicine published the DELIVER trial at the end of August 2022. This post is going to be a journal club breaking down the DELIVER trial and what change it may bring to our clinical practice.

The New England Journal of Medicine published the DELIVER trial at the end of August 2022. This post is going to be a journal club breaking down the DELIVER trial and what change it may bring to our clinical practice.
If you would like to listen to the article instead of read, play the audio file below!
Background/Overview
Title: Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction
Citation: Solomon SD, McMurray JJV, Claggett B, et al. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. NEJM 2022 Aug. https://www.nejm.org/doi/full/10.1056/NEJMoa2206286
Study purpose: To evaluate if dapagliflozin would reduce the risk of worsening heart failure (HF) or cardiovascular (CV) death among patients with a mildly reduced or preserved ejection fraction.
Background: Sodium-glucose cotransporter 2 inhibitors (SGLT2is) were originally approved as glucose-lowering agents for patients with type 2 diabetes mellitus (T2DM). The first SGLT2i, canagliflozin, was approved in 2013. Over the last decade, this class of medications has gained recognition for their efficacy in reducing the risk of death and other adverse outcomes in patients with HF reduced ejection fraction (HFrEF; EF <40%) and in patients with chronic kidney disease. The SGLT2is have shown benefit in these populations with and without T2DM.
Multiple mechanisms for SGLT2i benefit in the HFrEF population have been postulated. The role of SGLT2is in patients with HF preserved ejection fraction (HFpEF) remains limited to empagliflozin from the EMPEROR-Preserved trial published in October 2021. In this trial, empagliflozin was shown to lower the risk of hospitalization for HF regardless of the presence of T2DM.
The utility of SGLT2is in the HF population has several gaps considering the recent update to heart failure categories in the ACC/AHA/HFSA 2022 Guidelines.
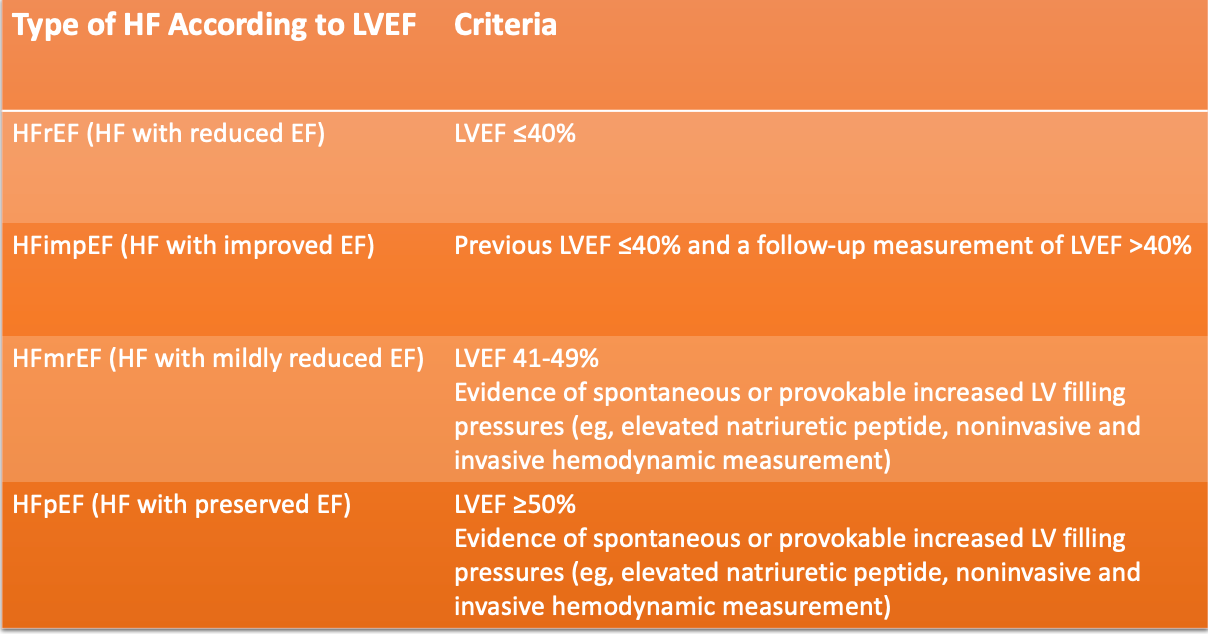
The DELIVER trial was designed to include a broader category of HF patients to further evaluate the utility of dapagliflozin in patients with HFimpEF, HFmrEF, and HFpEF.
Funding sources: Astra-Zeneca (manufacturers of dapagliflozin).
Methods
Study design: International, multi-center, phase 3, double-blind, randomized, event-driven trial; Aug 2018 - Dec 2020, 353 centers, 20 countries.
Intervention: Dapagliflozin 10 mg by mouth daily or placebo, in addition to usual therapy.
Patient population:
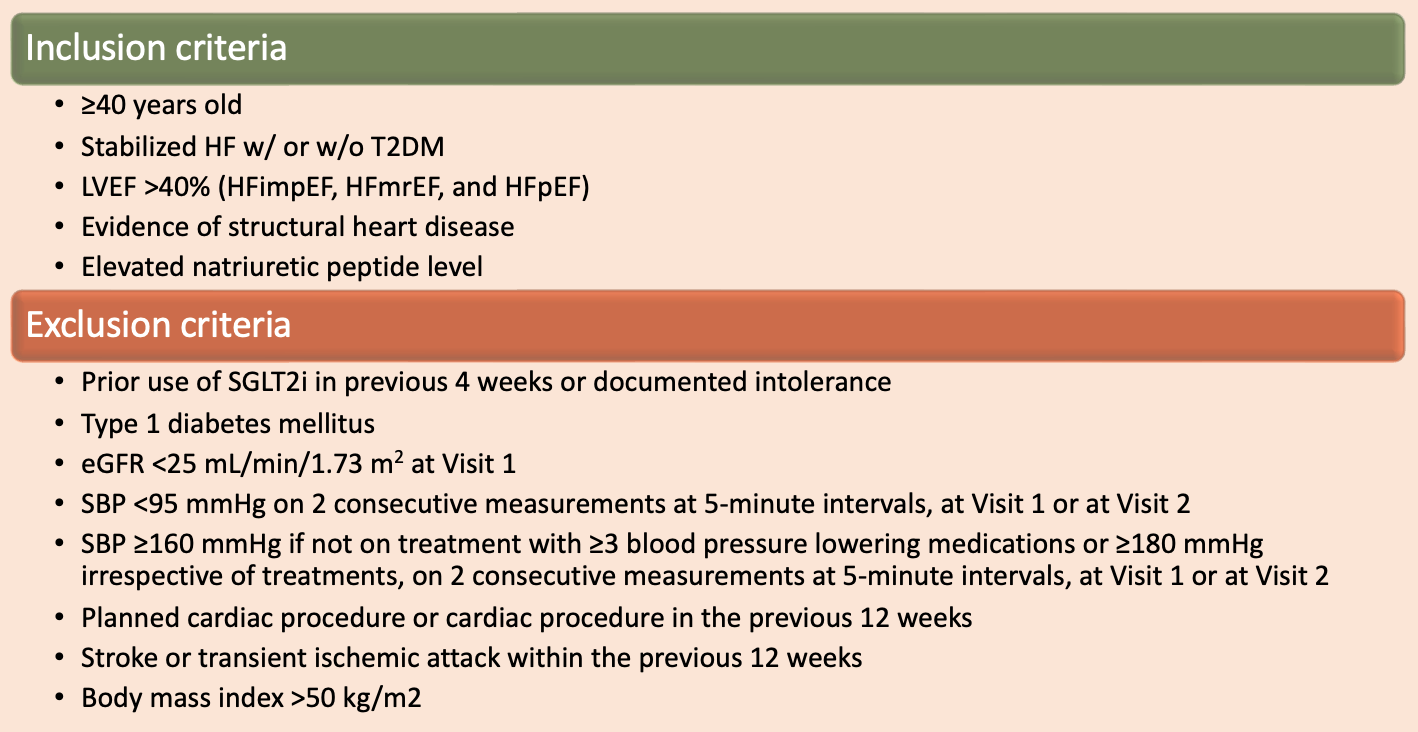
Outcome measures:
- Primary endpoints: Composite of worsening HF (defined as unplanned hospitalization for HF/an urgent visit for HF) or CV death.
- Secondary efficacy endpoints: Total number of worsening HF events and CV deaths, the change from baseline in the total symptom score on the KCCQ (Kansas City Cardiomyopathy Questionnaire) at month 8, CV death, and death from any cause.
- Safety endpoints: Serious adverse events, adverse events that led to discontinuation of dapagliflozin or placebo, and select other adverse events.
Statistical analysis: The primary outcome assessed on time-to-event analysis using Cox proportional-hazards model and stratified based on diabetes status.
- This was performed on the overall population (alpha set at 0.024) and in those patients with LVEF <60% (alpha set at 0.038).
- Power calculation: 6100 patients followed for a minimum of 13.5 months would provide a minimum of 1117 events for 93% power and hazard ratio of 0.80. This was for the overall population with the two-sided alpha of 0.024 mentioned above.
The authors did censor data collected at the time of coronavirus disease 2019 (COVID-19). This was performed in a separate sensitivity analysis.
Results
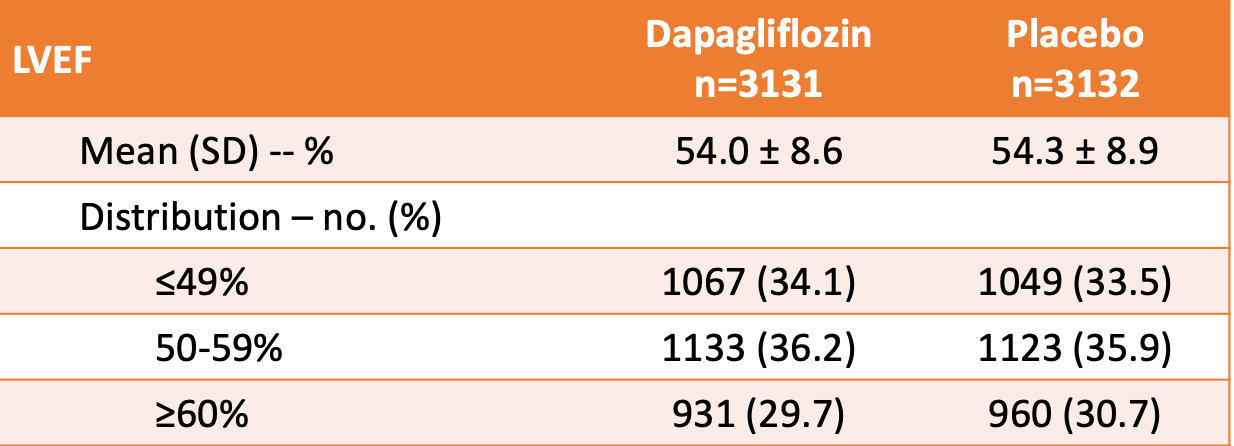
Baseline characteristics: 10,418 patients screened --> 6263 assigned to the intervention groups. The demographics between the groups were similar:
- Average age 72 years, 44% female, 71% White, 20% Asian, 75% NYHA Class II, 45% T2DM, estimated glomerular filtration rate of 61 mL/min/1.73 m^2.
- See table below for LVEF information.
- Dapagliflozin or the matching placebo pill was discontinued (for reasons other than death) in 14.2% and 14.1% of patients, respectively.
- Median duration of follow-up was 2.3 years.
Primary outcome results:
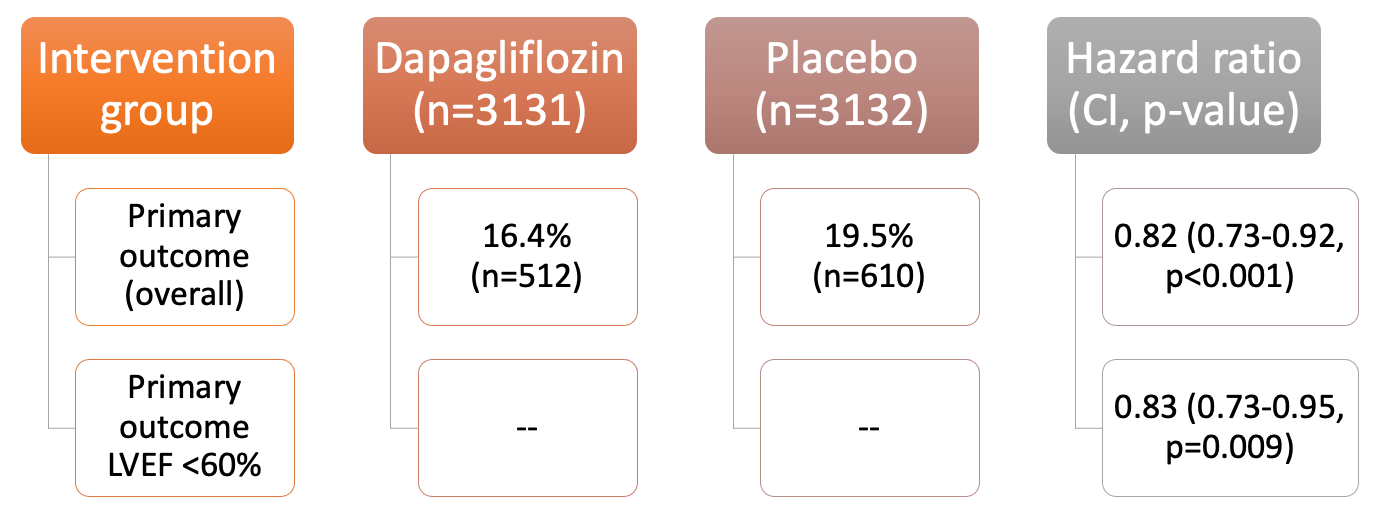
Secondary outcome results:
- The number of CV deaths and worsening HF events: lower in dapagliflozin group in the overall population (HR 0.77, CI 0.67-0.89, p<0.001).
- Incidence of components of the primary outcome favored dapagliflozin in the overall population and patients with LVEF <60% --> worsening HF (HR in the overall population, 0.79; CI, 0.69 to 0.91) and CV death (HR, 0.88; CI, 0.74 to 1.05) and death from any cause (HR, 0.94; CI, 0.83 to 1.07).
- Change from baseline to month 8 in KCCQ indicated a benefit with dapagliflozin.
- The effect of dapagliflozin on the primary outcome showed consistent in all pre-specified subgroups (most importantly, the presence or absence of T2DM).
- The COVID-19 sensitivity analyses also showed similar results.
Safety outcomes:
- Serious adverse events (including death) were reported in 43.5% and 45.5% of patients in the dapagliflozin and placebo groups, respectively.
- Rates of amputation (0.6% and 0.8%) and diabetic ketoacidosis (0.1% and 0%) were similar between the dapagliflozin and placebo groups, respectively.
Author's conclusions:
- Among patients with HF and a mildly reduced or preserved EF, dapagliflozin resulted in a lower risk of the composite worsening HF or CV death. These results provide further evidence to support SGLT2i use in patients with HF regardless of the presence of T2DM or LVEF.
Discussion
The use of dapagliflozin in patients with LVEF >40% was associated with an 18% reduction in the risk of worsening HF or CV death. The results of the DELIVER trial build upon the results from the EMPEROR-Preserved trial published in October 2021. These data bring into question the possibility of a class effect with the SGLT2is in HF patients regardless of LVEF and T2DM status.
It is important to note that the primary outcome was primarily driven by the reduction in HF hospitalizations, not by CV death. Therefore, it is appropriate to conclude that dapagliflozin reduces HF morbidity, but not mortality (that we know of at this point in time).
Study strengths
- Broader inclusion criteria compared to previous trials which increases internal and external validity.
- Subgroup analyses increase external validity and further generalizability to more populations.
- Analyzing each component of the composite primary outcome to determine which factor drove the overall outcome.
Study limitations
- Use of specific inclusion and exclusion criteria limits external generalizability.
- Less than 5% of patients enrolled were Black.
- Underpowered subgroup analyses (results should be interpreted with caution).
Despite the growing evidence for SGLT2i use, there are still numerous gaps that need to be researched to fully define the role of SGLT2is in patients with HF and LVEF >40%. Some of these gaps include use in the Black population and use in patients with HF due to cardiomyopathies.
Final Thoughts and Clinical Application
The outcomes of the DELIVER trial favoring dapagliflozin use in the HFmrEF, HFimpEF, and HFpEF populations provides reassurance for many health systems, insurance companies, and prescribers. My hospital, for example, only carries dapagliflozin on formulary. Prior to the DELIVER trial, the benefits of this specific SGLT2i was only theorized from the EMPEROR-Preserved trial which assessed empagliflozin use in a similar patient population. In addition, insurance companies may expand or adjust their formularies to include various SGLT2is as this may be a class effect of the medication. Prescribers can feel confident using any SGLT2i (or at least empagliflozin and dapagliflozin) in their HF patients depending on what the patient can afford and the formulary of their insurance/health system.
I am not surprised by the results of the DELIVER trial, and I think the replication of results from the EMPEROR-Preserved trial provides more robust data for our HF patients. There are not many therapies that reduce morbidity in patients with HF and a LVEF >40%. Now, the SGLT2i medications are an option for this patient population. In addition, these medications are relatively safe and provide multiple benefits outside of HF (potential weight loss benefits, T2DM control, blood pressure lowering, etc.).
As pharmacists, we can now potentially make evidence-based recommendations for our patients who fit the baseline demographics of the DELIVER and EMPEROR-Preserved trial. If a HF patient requires additional blood pressure lowering and does not present with worrisome risk factors relating to SGLT2i use (think common side effects from this class of medications: urinary tract infections, hypoglycemia, orthostatic hypotension, diabetic ketoacidosis, poor renal function), then a great option would be to start an SGLT2i!
Extra credit question: How do you interpret a hazard ratio (HR)?
- An HR is the result of comparing the hazard function of a group exposed to a variable with the hazard function of a group not exposed to that variable. As with any type of ratio, an HR of 1 means a lack of difference, an HR greater than 1 suggests an increased risk for the exposed group, and an HR below 1 suggests a decreased risk for the exposed group. In the DELIVER trial, the exposed group is patients who received dapagliflozin, and the non-exposed group is the patients who received placebo.
- In the discussion above, I underlined a portion of the first sentence: "The use of dapagliflozin in patients with LVEF >40% was associated with an 18% reduction in the risk of worsening HF or CV death." Noticed that it is an 18% reduction in the risk, not an absolute 18% reduction of the primary outcome all together. This is because an HR is comparing the risk (aka hazard) of an event in one group compared to another. In the DELIVER trial, the primary outcome resulted with an HR of 0.82 (CI 0.67-0.89) between dapagliflozin and placebo. Since the HR is less than 1, it suggests that there is less risk of the primary outcome in the dapagliflozin group compared to placebo. To find the risk reduction (because we are talking about a risk here), you can take 100 x (1-HR) to find that reduction in risk.
100 x (1 - 0.82) = 100 x (0.18) = 18%
- Notice the confidence interval (CI) of 0.67-0.89. Since we are talking about a ratio, if the CI includes 1, then there is a possibility that no difference exists between patients treated with dapagliflozin and placebo. We know that with a ratio of 1, the top and bottom number of the ratio are the same. Think about a ratio of apples to oranges where you have 5 apples and 5 oranges. The ratio is 5/5 which equals 1 --> no difference between the amount of apples and oranges.

