What Causes a Heart Attack?
Cardiovascular disease has been a major cause of death in the United States for nearly a century, and according to the American Heart Association, around 80% of cardiovascular diseases including heart disease and stroke are preventable.

Author: Jarret Morgan
Editor: Brentsen Wolf, PharmD
I have a family history of vascular incidence, and thus, cardiovascular health is incredibly relevant and important to me. Cardiovascular disease has been a major cause of death in the United States for nearly a century, and according to the American Heart Association, around 80% of cardiovascular diseases including heart disease and stroke are preventable. It's sad to hear that so many emotional, life-altering events could have been mitigated for family members and friends. One of the highest contributors to cardiovascular disease is atherosclerosis. Atherosclerosis is a disease process that can potentially lead to the occlusion (blocking) of important blood vessels like the ones that supply your heart and brain with oxygen and nutrients. In this post, I’ll summarize the pathophysiology of atherosclerosis and cover different clinical markers used to assess individual risk.

With the topic of atherosclerosis, it's necessary to mention the solubility of molecules with varying polarities, lipid metabolism, and lipid/cholesterol transportation. Maybe you've heard the saying “like dissolves like”, and this is a nice generalization of how nonpolar and polar molecules do not mix. This is the same basic principle behind pouring water and oil into a container to watch them separate from each other. Lipids are nonpolar molecules whilst water is a polar molecule, so they don't mix well. Considering your body is mostly water, lipid metabolism and transport can get pretty complicated. This video is a good example of how it works (the card is a lipid).
Lipid metabolism begins with lingual lipase (an enzyme found in the mouth) and finishes in the small intestine via emulsification by bile salts into smaller components such as glycerol, monoglycerides, and a mixture of fatty acids. These nonpolar molecules quickly form a micelle (a structure with internal nonpolar parts and external polar parts), which separates its nonpolar components from the polar surroundings. This newly formed micelle can interact in the polar solution and will diffuse across the plasma membrane of your small intestine’s epithelial cells to be resynthesized into new triglyceride molecules. The epithelial cells will repackage the newly synthesized triglycerides with cholesterol, apoB48 (a protein component), and other lipids to form chylomicrons or ULDLs (ultra low-density lipoproteins) in order to make them soluble in the polar solution that is your bloodstream.
A chylomicron is a lipoprotein with a water-soluble structure similar to micelles. Chylomicrons will exit the intestinal cells through exocytosis and make their way to the liver for processing and alteration. Moving these lipid components in the bloodstream is similar to moving candy into the movie theatre. The protein coat is your girlfriend’s purse, and the lipids are sour patch kids.
The liver processes the contents of the chylomicrons and makes alterations to the surface protein components that enclose the lipids. It generates several new lipoproteins, some of which include very low-density lipoproteins (VLDLs) which transport triglycerides to muscle and adipose tissues, low-density lipoproteins (LDLs) which deliver cholesterol to peripheral tissues, and high-density lipoproteins (HDLs) which absorb excess cholesterol in circulation and bring them back to the liver. Another important alteration to surface proteins by the liver is the addition of apoprotein B100 (apoB100) which is a structural protein found on every VLDL, IDL (intermediate-density lipoprotein), LP(a) (lipoprotein little a), and LDL particle, but not HDL particles.
So, what does all this lipid talk have to do with cardiovascular disease? Atherosclerosis begins with lipoproteins (primarily LDL due to an extended plasma residence time) squeezing between the endothelial cell layer of your blood vessel’s tunica intima (this is the internal layer of blood vessels) to eventually form a fatty (lipid) streak/lesion.
I should clarify that although cholesterol gets a bad rap, it actually plays a number of incredibly important roles in your body. For instance, cholesterol helps maintain the structural integrity of your cells' plasma membrane with a cholesterol molecule for nearly every phospholipid. This is true for most of the trillions of cells that you have. Not just that, but cholesterol is also a major constituent of different hormones that regulate various processes in your body. A few examples include estrogens, androgens, glucocorticoids, mineralocorticoids, and more. So, you do need cholesterol for your body to function to the best of its ability.

Jumping back to atherosclerosis, problems occur when an accumulation of cholesterol-containing particles (primarily LDL) is found beneath the endothelial cell layer. This isn’t necessarily an immediate danger, but as soon as damage occurs to the endothelium, the situation can escalate. When cells get damaged, just like when you get a small scratch on your skin, inflammation occurs to supply the area with increased blood flow in hopes to rapidly kickstart the healing process. The increased blood flow will deliver white blood cells, plasma proteins, and biochemical mediators to the site of injury. A byproduct of the inflammation is the generation of toxic free radicals which oxidize the accumulated lipoprotein particles. Some of the delivered white blood cells are monocytes. Monocytes can effectively squeeze through the endothelium through a process called diapedesis, then differentiate into macrophages which are particularly good at phagocytizing (engulfing) any debris or foreign bodies. These macrophages will phagocytize the oxidized lipoprotein particles, and when the macrophages become saturated with these oxidized particles, they are referred to as foam cells due to having a "foamy" appearance.
When there are significant numbers of foam cells, a fatty streak will develop. These fatty streaks will increase the number of toxic free radicals, recruit T-cells which can lead to autoimmunity, and secrete inflammatory mediators which all further damage the vessel wall. Meanwhile, macrophages will release growth factors to promote smooth muscle cell proliferation which will lead to the production of collagen over the fatty streak to produce a fibrous plaque. This fibrous plaque may calcify and protrude into the luminal space to obstruct blood flow to tissues.

Depending on the location, this may manifest into symptoms of angina, headaches/facial pain, or muscle pain during activity, which stops when you're at rest. Many of these plaques are prone to rupturing and initiating a clotting cascade due to the exposure of damaged underlying tissue. This hypothetical blood vessel already has a decreased luminal space, but now with the thrombogenesis process in full blast, the potential to occlude this blood vessel has increased dramatically. Why is this important? Well, the occlusion of a blood vessel results in ischemia (lack of oxygen) in the tissues it normally supplies. Without oxygen, cell death will result, and depending on the location of these cells you may have an incoming heart attack or stroke, both of which can kill you.
Sound scary? That's because it is.
So, who is most susceptible to developing atherosclerosis? While there is no way to intrinsically know that someone is or isn’t susceptible, co-morbidities such as obesity, hypertension, diabetes, and smoking tend to increase risk. Genetics and blood work can also help provide some clarity to individual susceptibility.
Some of the blood tests include measurements of LDL-P, LDL-C, non-HDL-C, and apoB. LDL-P measures serum LDL particle number and LDL-C measures (or calculates) the mass of serum LDL cholesterol. Both LDL-P and LDL-C will be important metrics to help determine an individual’s relative particle size and density. There are four separate categories that you may be placed in given your blood test results: high LDL-C/high LDL-P, low LDL-C/low LDL-P, high LDL-C/low LDL-P, and low LDL-C/high LDL-P. Your healthcare provider will be able to assess the given lab values to determine a more individualized risk. See below a graph from Cromwell et al. which provides a look into EFS (event-free survival) over time for different cholesterol categories. Being higher up on the y-axis equivalates to fewer deaths and increased survival.
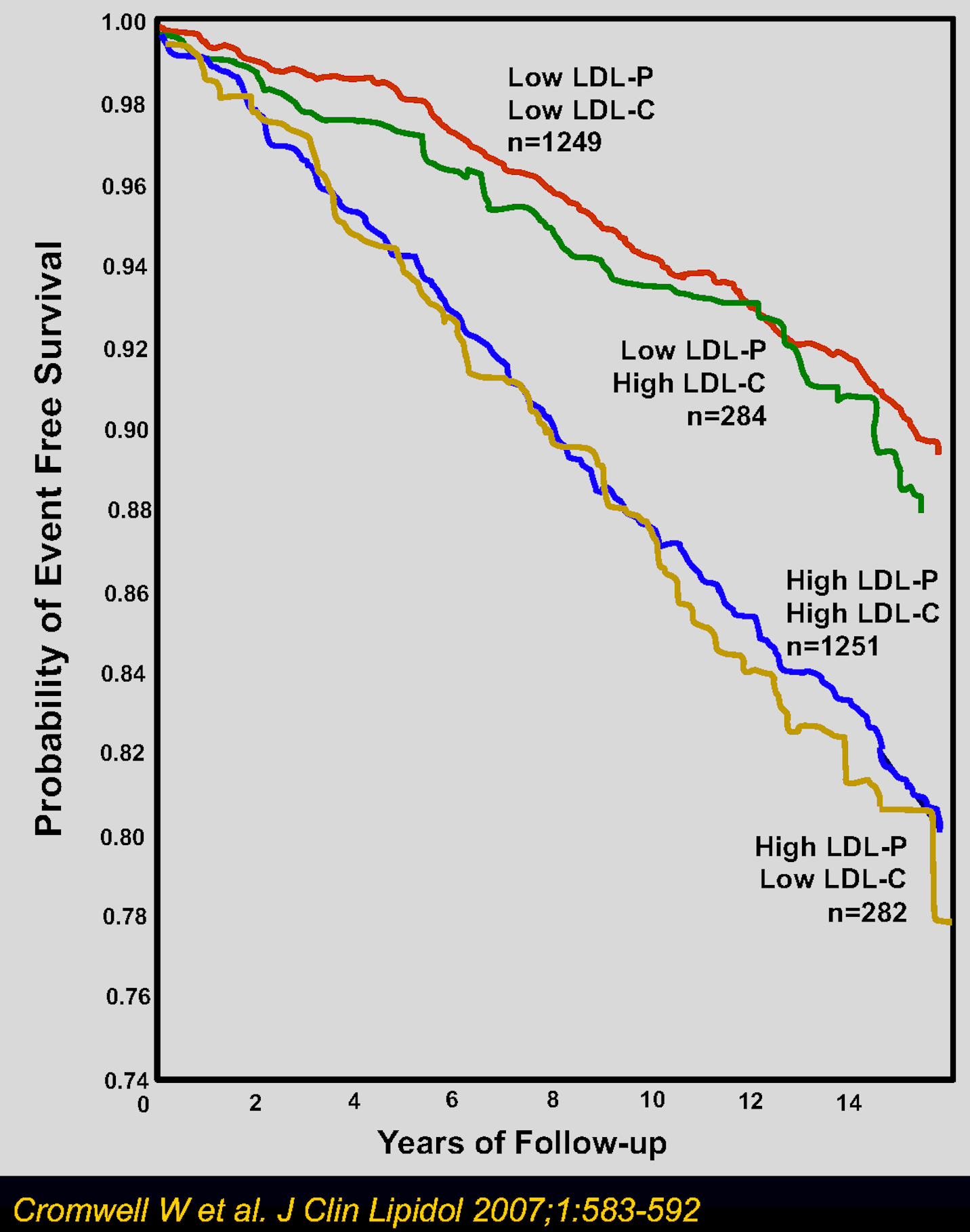
It seems that the smaller and denser LDL particles are associated with a higher degree of risk. Perhaps these smaller particles can squeeze their way under the endothelial cell layer more readily, which makes sense compared to a larger particle.
Non-HDL-C is a measure of all the cholesterol in circulation minus HDL cholesterol, while apoB measures the total number of apoB bearing particles. Both of these lab values are comparable, but non-HDL-C will be more specific to circulating cholesterol outside of HDL lipoproteins and apoB will be more specific to the number of atherogenic particles in circulation. Remember, there is only one apoB found on any of these particles (1 apoB = 1 particle). Around 90% of circulating apoB particles are LDLs, so apoB measurements would primarily be indicative of an LDL particle count but would still include other potentially atherogenic particles. LDL particles are more populous in the bloodstream and are primary contributors to fatty streak/lesion formation.
So, maybe you'll find out that you are at high risk, or maybe you’re a bit of a health fanatic and want to improve your stats as much as possible for longevity’s sake. Naturally, the most common advice given is to eat a balanced diet, lose weight, keep your hypertension/diabetes in check, quit smoking, and exercise. Some people may be more genetically at risk as displayed earlier in this article with smaller, more dense LDL particles. That alone may help explain why some people have long family histories of cardiovascular disease.
I hope that you found this topic as interesting as I do. Remember, this information can be crucial in prolonging good quality of life for patients and families. Be a conduit and share this information to help people like my grandfather quit smoking or understand the labs they get from their physicians.
Author Bio: Jarret Morgan is a pre-pharmacy student at the Southern Illinois University of Edwardsville and a part-time inpatient pharmacy technician at a local hospital. He aspires to one day attain a fellowship in the pharmaceutical industry where he can broaden his skillset and make a difference at the macro-scale. In his free time, Jarret enjoys exercising, playing sports/video games with his wife, and spending time with his grandfather.



