Engineering in Pharmacy
Explore how collaboration between pharmacy and engineering drives innovation in drug delivery, device design, and patient-centered care.

Author: Zacheus Carr, PharmD Candidate 2029
Editor: Kristen Lindauer, PharmD, BCPS, AAHIVP
Introduction
Pharmacy and bioengineering share the same ultimate goal: improving patient outcomes and enhancing quality of life. Yet, collaboration between the two fields has not kept pace with rapid advances in medicine and technology. Given the rise of biologics, advanced drug delivery devices, and personalized medicine, the influence of engineering on pharmacy is more significant than ever. Every day, pharmacists work with engineered products such as inhalers, insulin pens, patches, and controlled-release tablets, along with the packaging and manufacturing systems that bring these products to patients (see Table 1 for a breakdown of these engineered products).
As frontline providers, clinical pharmacists hold unique insight into what makes these products practical, including how easily they can be stored, dispensed, and explained to patients. They also hear firsthand the challenges patients face when using these products, often receiving the brunt of patient complaints. From educational settings to corporate environments, strengthening connections between pharmacists and the engineers and scientists who design these products would not only improve pharmacist–patient interactions by equipping pharmacists to address deeper questions about product design, but would also enhance the design process itself by ensuring that patient and pharmacist needs are fully considered.
Table 1: The graphics in the left column of the table illustrate the key properties of common engineered pharmaceutical products, including metered-dose inhalers1, insulin pens2, transdermal patches3, and controlled-release tablets4,5. The right-hand column summarizes the underlying engineering principles and mechanisms of action for each corresponding product.
Where Engineering and Pharmacy Overlap Today
Many important medical products on the market today were created through collaboration between medical professionals and engineers. One clear example is the transdermal patch. Although the idea of delivering drugs through the skin had previously existed, it was not until 1971 that biochemist and entrepreneur Alejandro Zaffaroni and his company, Alza, designed a patch that applied engineering principles and pharmacokinetics to control drug delivery. Their innovation, a patch containing both a reservoir and a rate-limiting membrane6, made it possible to regulate drug input into the bloodstream while reducing variability from the skin. Since then, teams of engineers, scientists, and clinicians have advanced the technology into the wide range of patches available today.
Currently, four main types of transdermal patches are in use:
- drug-in-adhesive
- reservoir, matrix
- micro-reservoir
- microneedle patches
- solid
- hollow
- coated
- dissolving
Another transformative medical invention that emerged from a multidisciplinary team is the metered-dose inhaler (MDI). Its origin traces back to 1956, when a 13-year-old girl with asthma asked why her medication could not be delivered like her hairspray. Her father, Dr. George Maison of Riker Laboratories, posed the question to his chemists, who collaborated with colleagues in the cosmetics industry and engineers working on propellant technology8. Within a year, they had adapted aerosol and valve designs originally intended for perfumes into the first portable rescue inhaler. Chemists such as Charlie Thiel refined the formulation through hundreds of experiments. He ultimately created a suspension system that allowed drugs, like hydrocortisone, to be reliably delivered as a fine mist to the lungs. This breakthrough provided the first convenient, effective, and user-friendly inhaler for asthma patients. This also laid the foundation for today’s pressurized MDIs, which remain the most widely used inhalation devices worldwide9.
Many other transformative products also originated from the collaboration of medical and engineering experts. Drug-eluting stents, for instance, were pioneered through the combined expertise of cardiologists, biomedical engineers, and pharmacologists. Insulin pens and autoinjectors were created by teams of clinicians working alongside mechanical and chemical engineers, while insulin pumps were advanced through the joint efforts of engineers, pharmacists, biochemists, and physicians. Together, these examples reinforce the pattern that meaningful progress in drug delivery and medical technology consistently arises when diverse professional perspectives intersect.
Why Collaboration is Key
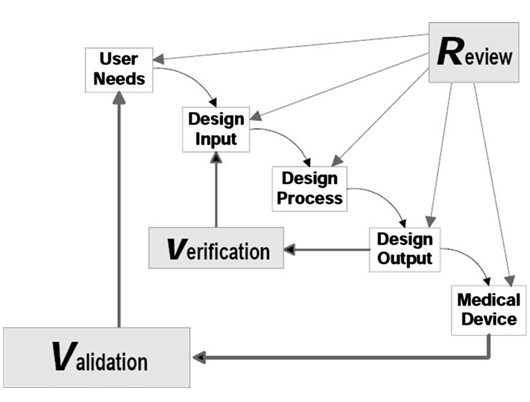
It is clear that multidisciplinary teams are the key to success when it comes to innovation in both medical devices and drug products. For medical devices, professionals must follow the FDA waterfall design process. Adhering to this framework with proper documentation allows for a more efficient review process (Figure 1).
The first step is defining the user needs. Though this step may seem straightforward, it is critically important. One of the most common reasons medical devices fail is due to poor demand and lack of adoption. A device failing for this reason represents a devastating mistake, but one that can be avoided if patient and provider needs are carefully analyzed at the beginning of development. While this may increase upfront costs, it is ultimately beneficial for companies, ensuring that investments are directed only toward products that will be used in practice.
Drug product development, while not guided by a parallel FDA diagram like that of medical devices, follows a well-established process of its own. Typically, a company begins with a cost-benefit analysis to determine whether research in a particular therapeutic area is worthwhile. Alternatively, a grant review committee could undergo a similar process to decide whether to fund research in that area. Once the project is deemed significant, potential drug candidates are screened for desirable properties, optimized, tested in animals, and advanced into Phases 1–3 of clinical trials in humans to demonstrate safety and efficacy. The most common reasons drugs fail include poor pharmacokinetics, lack of intrinsic activity, toxicity, and lack of market10. As with devices, failure due to lack of market demand is especially costly and can often be avoided with thorough upfront analysis. Ultimately, ensuring that a new therapy not only addresses an unmet medical need, but will also be adopted by patients and clinicians, is essential to long-term success.
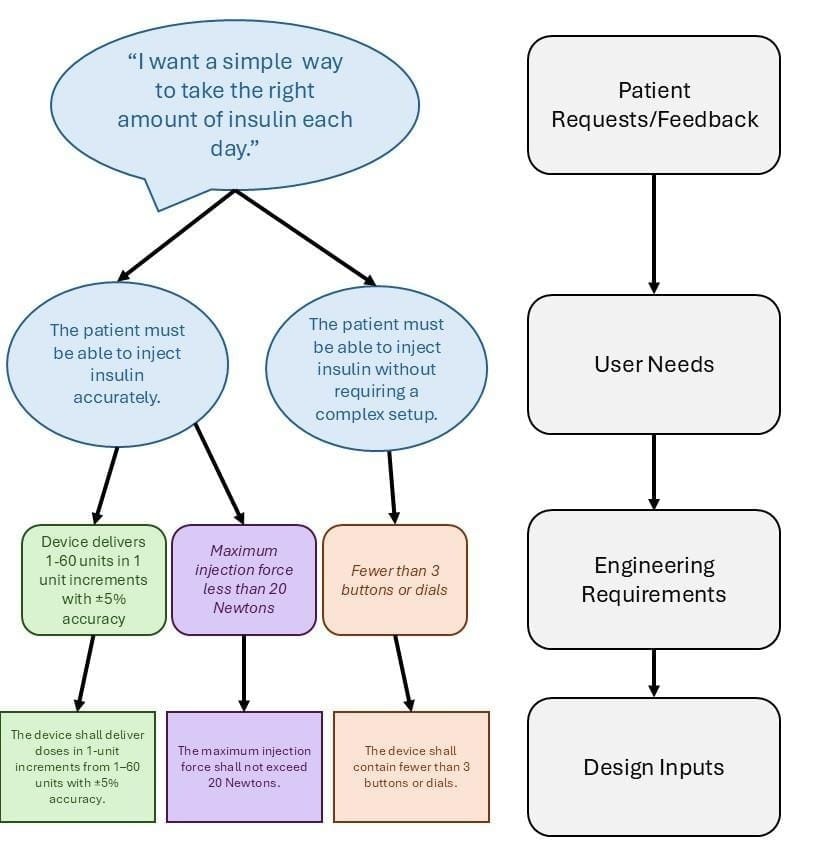
Although the pathways for devices and drugs differ, they share a common starting point: identifying and addressing real-world patient needs. In terms of personnel, this is where pharmacists, physicians, and other medical professionals are especially valuable. Clinicians interact with patients daily, giving them a strong understanding of what patients need and what is required for a device or medication to be successful. After defining the user needs in the medical device development process, the subsequent steps involve translating those needs into engineering requirements (informal technical specifications that can be measured). As these requirements are refined and agreed upon, they are then formalized as design inputs, which are necessary for FDA design control.
From this patient statement, two user needs can be identified:
- “The patient must be able to inject insulin accurately,” and
- “The patient must be able to inject insulin without requiring complex setup.”
The corresponding engineering requirements for the accuracy user need could include: “Device delivers 1–60 units in 1-unit increments with ±5 % accuracy” and “Maximum injection force less than 20 Newtons.” For the simplicity-related user need, the corresponding engineering requirement might be “Fewer than 3 buttons or dials.” Once finalized, these requirements are formalized as design inputs: “The device shall deliver doses in 1-unit increments from 1–60 units with ±5 % accuracy,” “The maximum injection force shall not exceed 20 Newtons,” and “The device shall contain fewer than 3 buttons or dials.” This whole process is diagramed below in Figure 2.
After defining the design inputs, the next step in the process is concept generation. Many companies follow the principle that “quantity breeds quality,” aiming to approach the problem from every possible angle and generate a wide range of solutions. Concepts are then refined, combined, and narrowed down, taking the strongest elements from each idea to create the best possible design. The more unique the perspectives and diverse backgrounds during this stage, the better the outcome tends to be. This is why multidisciplinary teams of pharmacists, engineers, scientists, and physicians are so effective at driving innovation.
Testing begins once a design concept has been selected and translated into design outputs. The first step is verification testing. This measures whether the product meets the engineering requirements. These are often in vitro tests that generate numerical results, which can be directly compared to the predetermined design inputs. If the product fails verification, the design must be revised until the requirements are satisfied. After verification, the product advances to validation testing to be evaluated by real users. This step not only ensures that the product is safe and effective, but also that it is practical and acceptable for patients. If a product fails safety or efficacy standards, it will not receive FDA approval. Yet, even after approval and entry into post-market surveillance, products that are overly complex or inconvenient may still fail due to poor patient or provider adoption. Because developing a medical product is a long and costly process, failure at this stage represents a catastrophic loss, underscoring why the first step – clearly defining user needs with input from healthcare professionals and patients – is so critical.
Promoting More Interdisciplinary Work
With interdisciplinary teams proving so effective in advancing healthcare, it seems logical that educational programs should provide more opportunities for students to train in these settings. According to the Accreditation Council for Pharmacy Education (ACPE) accreditation standards, all pharmacy schools must include interprofessional education (IPE) experiences in their curricula, as outlined under Sections 2.1.h and 2.311. However, these requirements are typically limited to collaborations with other clinical professions such as physicians, dentists, and nurses. Few programs create opportunities for pharmacy students to engage with engineering or PhD-level research students, even though such experiences would be invaluable in teaching future clinicians and researchers how to collaborate effectively from the earliest stages of their careers.
Such experiences would help both professions better understand the full process from lab bench to patient. They could also build lifelong connections, enabling future clinicians to reach out to researchers and engineers with problems observed in patient care. This could spark conversations that might lead to the development of new medical products.

For pharmacy students, early exposure to this type of collaboration would provide a perspective that supports more informed decisions when administering novel therapies. It would also better prepare pharmacy students for roles outside of traditional practice, as more licensed pharmacists transition into industry and other non-traditional settings each year12. At the same time, engineering students would gain valuable insight into the realities of clinical practice, equipping them for careers that intersect with healthcare or even for the expanding field of clinical engineering.
These workforce trends – pharmacists entering the industry, and engineers taking on clinical roles – demonstrate that companies and healthcare systems already recognize the value of interdisciplinary teams and the need for a cohesive process from idea generation to patient care. Educational programs should follow this lead by creating more opportunities for students to engage in cross-disciplinary training. Some schools are already moving in this direction; for example, Washington State University’s College of Pharmacy offers a dual PharmD and Master’s in Engineering Management degree designed to prepare students to collaborate effectively with engineers. Expanding initiatives like this across more pharmacy schools would ensure that future pharmacists graduate with not only strong clinical expertise but also the collaborative skills necessary to shape the next generation of therapies and medical devices.
Conclusion
The present marks an exciting era of rapid technological advancement, innovation, and improved medical outcomes. With pharmacists, physicians, engineers, and researchers contributing across the medical landscape, the greatest limitation to progress lies not in capability but in communication. When collaboration across disciplines is weak and fragmented, even the most promising ideas may fail to reach or satisfy patients in need. Preparing future professionals to think collaboratively from the start will help ensure that advances in technology translate into advances in care. Strengthening interdisciplinary collaboration between pharmacy and engineering will allow medical innovation to continue fulfilling its highest purpose: improving patient quality of life.
About the Author

References
[1] Drugs.com. (2025, October 5). How to use a metered-dose inhaler. https://www.drugs.com/cg/how-to-use-a-metered-dose-inhaler.html
[2] Novo Nordisk. (2005, May 17). NovoPen®3 PenMate instruction manual (Label No. 019938 S-037). U.S. Food and Drug Administration. https://www.accessdata.fda.gov/drugsatfda_docs/label/2005/019938s037lbl.pdf
[3] U.S. Food and Drug Administration. (2019, November). Transdermal and Topical Delivery Systems – Product Development and Quality Considerations https://www.fda.gov/media/132674/download
[4] Kumar, M., Kumar, U., & Singh, A. (2022). Therapeutic nanoparticles: Recent developments and their targeted delivery applications. Nano Biomedicine and Engineering, 14(1), 38–52. https://doi.org/10.5101/nbe.v14i1.p38-52
[5] Adepu, S., & Ramakrishna, S. (2021). Controlled drug delivery systems: Current status and future directions. Molecules, 26(19), 5905. https://doi.org/10.3390/molecules26195905
[6] Pastore, M. N., Kalia, Y. N., Horstmann, M., & Roberts, M. S. (2015). Transdermal patches: History, development and pharmacology. British Journal of Pharmacology, 172(9), 2179–2209. https://doi.org/10.1111/bph.13059
[7] Wong, W. F., Ang, K. P., Sethi, G., & Looi, C. Y. (2023). Recent advancement of medical patch for transdermal drug delivery. Medicina, 59(4), 778. https://doi.org/10.3390/medicina59040778
[8] Contract Pharma. (2017, April 19). Inventing the MDI: A history in modern inhalation therapy. https://www.contractpharma.com/exclusives/inventing-the-mdi-a-history-in-modern-inhalation-therapy/
[9] Steiropoulos, P., Bakakos, P., Hatziagorou, E., Katsaounou, P., Loukides, S., Papaioannou, A., Porpodis, K., Samaras, K., Tzouvelekis, A., Kalafatakis, K., & Kostikas, K. (2021). The present and future of inhalation therapy for the management of obstructive airway diseases: Emphasis on pressurized metered-dose inhalers. Pneumon, 34(1), 1–13. https://doi.org/10.18332/pne/144614
[10] Sun, D., Gao, W., Hu, H., & Zhou, S. (2022). Why 90% of clinical drug development fails and how to improve it? Acta Pharmaceutica Sinica B, 12(7), 3049–3062. https://doi.org/10.1016/j.apsb.2022.02.002
[11] Accreditation Council for Pharmacy Education. (2025). Accreditation standards and key elements for the professional program in pharmacy leading to the Doctor of Pharmacy degree (“Standards 2025”). Chicago, IL: Author. https://www.acpe-accredit.org
[12] University of Wisconsin School of Pharmacy. (2025, June 16). Emerging trends from the 2024 National Pharmacy Workforce Study. https://pharmacy.wisc.edu/2025/06/16/emerging-trends-from-the-2024-national-pharmacy-workforce-study/

