IMforte Journal Club
Overall, this trial was well-designed and provides promising evidence of OS and PFS benefit for patients with extensive-stage SCLC, a historically aggressive disease with poor outcomes

Welcome back to RxTeach. I am excited to share my first journal club on this platform and hope to bring you valuable insights into the treatment landscape for extensive-stage small-cell lung cancer! 😄
The IMforte clinical trial was published in The Lancet in June 2025. Today, we will be breaking down the trial and showcasing its impact on clinical oncology treatment.
Background/Overview
Title: Efficacy and safety of first-line maintenance therapy with lurbinectedin plus atezolizumab in extensive-stage small-cell lung cancer
Citation: Paz-Ares L, Borghaei H, Liu SV, et al. Efficacy and safety of first-line maintenance therapy with lurbinectedin plus atezolizumab in extensive-stage small-cell lung cancer (IMforte): a randomised, multicentre, open-label, phase 3 trial. Lancet. 2025;405(10495):2129-2143
Objective: Assess the efficacy and safety of adding lurbinectedin to the current standard-of-care, atezolizumab, in the maintenance treatment of ES-SCLC in patients whose disease had not progressed following induction therapy with atezolizumab, carboplatin, and etoposide


There were additional secondary and exploratory endpoints, but they will not be discussed to remain concise*
- Safety was assessed in a safety analysis set, comprised of patients who had received at least 1 dose of lurbinectedin or atezolizumab, and analyzed according to the treatment received
Methods:
Participants:
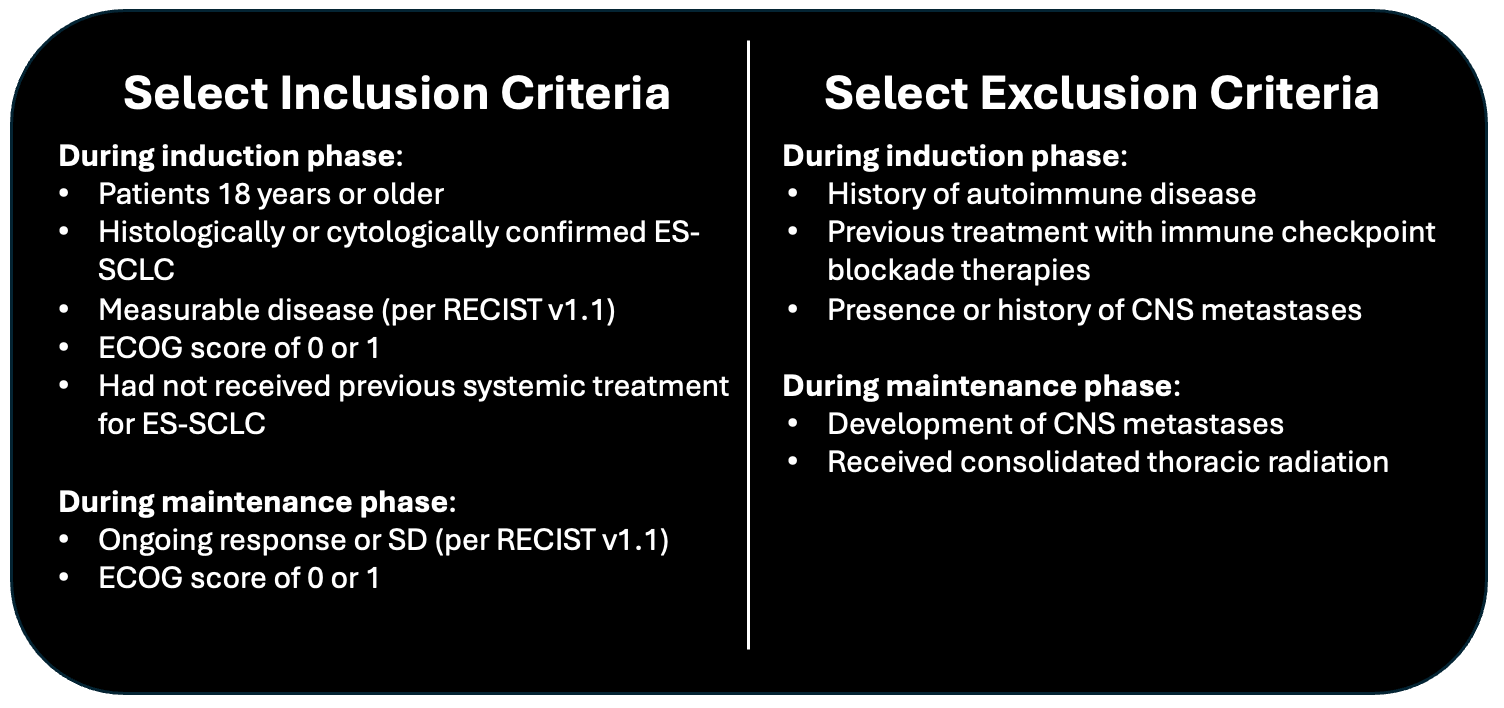
Design: Multicenter, international, randomized, open-label phase 3 trial. Screening occurred between November 2021 and January 2024. Patients were enrolled in the maintenance phase from May 2022 to April 2024. Data cutoff July 29, 2024
- Eligible patients were randomly assigned (1:1) to maintenance therapy using a permutated block schedule (block size 4)
- Randomization was stratified by ECOG performance status at maintenance baseline (0 or 1), lactate dehydrogenase at maintenance baseline (≤ upper limit of normal [ULN] or >ULN), the presence of liver metastasis at induction baseline (yes or no), and previous receipt of prophylactic cranial irradiation (yes or no)
Treatments: During the induction phase, all study participants received four 21-day cycles of atezolizumab, carboplatin, and etoposide. Atezolizumab (1200 mg) was given IV every 3 weeks, while both carboplatin and etoposide were administered per institutional practices
Following the induction phase, eligible participants were randomized to receive either atezolizumab (1200 mg) + lurbinectedin (3.2 mg/m2) or atezolizumab (1200 mg) monotherapy administered IV every 3 weeks until the occurrence of unacceptable toxic effects, disease progression, or withdrawal of study participation
- Study treatment beyond disease progression was not permissible per protocol
- Patients in the lurbinectedin + atezolizumab group received prophylactic granulocyte colony-stimulating factor G-CSF unless contraindicated, and anti-emetic premedication per institution protocol
- Two dose reductions were allowed for lurbinectedin from 3.2 mg/m2 to 2.6 mg/m2, then from 2.6 mg/m2 to 2.0 mg/m2; reescalation was not permitted
- Atezolizumab dose modifications were not permitted
- If treatment in the lurbinectedin + atezolizumab group had to be temporarily interrupted or permanently discontinued to manage toxicity, either agent could be interrupted or discontinued independently from the other
- Crossover of treatment groups was not permitted
Duration: Median follow-up was 15.0 months (IQR 8.6 – 18.3)
Statistical Analysis: The study was designed to randomize 450 participants, which was deemed enough to demonstrate efficacy
- They had approximately 85% power with a two-sided significance level of 0.049 to detect a hazard ratio (HR) of 0.71 for OS in the full analysis set
- It was estimated that at the OS interim analysis, approximately 392 IRF PFS events would have occurred, providing 99%+ power to detect an HR of 0.5 for IRF PFS at a two-sided significance level of 0.001
- Primary endpoints were compared between treatment groups based on a stratified log-rank test using the previously mentioned stratification factors during randomization
- HRs and 95% CI for both PFS and OS were estimated using a stratified Cox regression model
- Kaplan-Meier curves were utilized to calculate median PFS and median OS
Results:
660 participants were enrolled in the induction phase at 96 sites in 13 different countries. Following induction, 484 (73%) of the 660 participants were randomly assigned to maintenance treatment
- Baseline characteristics were generally well-balanced between groups, with the exception of age, where the lurbinectedin + atezolizumab group had a higher proportion of younger patients (<65 years of age)
- 63% of participants were male
- 80% of participants were from Europe and the Middle East; 13% from Asia-Pacific
- 82% were white; 13% Asian
- 64% were previous smokers; 33% current smokers
- 40% had liver metastases at induction baseline
- 85% had previous prophylactic cranial irradiation
- 43% had an ECOG score of 0 at maintenance baseline; 57% an ECOG score of 1
- 73% had an LDH of ≤ULN at maintenance baseline
- In response to induction therapy, 88% had either a complete or partial response; 11% had stable disease; 1% had progressive disease
At the clinical cutoff, 174/242 (72%) of the lurbinectedin + atezolizumab group and 202/241 (84%) of patients in the atezolizumab monotherapy group had disease progression per IRF assessment or had died
Primary Outcomes:
Median IRF PFS: 5.4 months vs 2.1 months; stratified HR 0.54 (95% CI, 0.43 to 0.67); stratified p-value <0.0001
Median OS: 13.2 months vs 10.6 months; stratified HR 0.73 (95% CI, 0.57 to 0.95); stratified p-value 0.017
Specified Secondary Outcomes:
Investigator-assessed median PFS: 5.4 months vs 2.7 months; stratified HR 0.55 (95% CI, 0.45 to 0.68)
Objective Response: 34/175 (19%) vs 19/182 (10%) had a confirmed objective response per IRF assessment compared to the maintenance baseline scan. The between-group difference in ORR was 9% (95% CI 1 to 17)
Median duration of response: Assessed by the IRF was 9.0 months (95% CI 5.5 - not estimable) in the lurbinectedin + atezolizumab group and 5.6 months (4.2 - not estimable) in the atezolizumab group
Safety:
All-cause adverse events: 97% vs 81%
Adverse events considered related to any study treatment by the investigator: 83% vs 40%
Grade 3-4 adverse events: 38% vs 22%
Most common grade 3-4 adverse events were hematological toxicity: anemia (8% vs 1%), decreased neutrophil count (7% vs 0%), decreased platelet count (7% vs 0%), neutropenia (5% vs <1%), and thrombocytopenia (5% vs <1%)
Grade 3-4 treatment-related adverse events: 26% vs 6%
Serious adverse events: 31% vs 17%; those that occurred in more than 1% of patients in either group were pneumonia (2% vs 3%), dyspnea (2% vs 2%), respiratory tract infection (2% vs <1%), decreased platelet count (2% vs none), and febrile neutropenia (2% vs none).
Treatment-related serious adverse events: 12% vs 4%
Deaths due to adverse events: 5% vs 3%
Strengths:
- Multicenter and international design
- Independent review facility assessed primary endpoint
- Intention-to-treat analysis for efficacy outcomes, including all those randomly assigned to maintenance treatment, regardless of whether they received their assigned study treatment
- Randomization with a permutated block schedule allowed for balanced treatment groups throughout enrollment
- Stratification factors are well validated, preventing confoundment from impacting study outcomes
Limitations:
- An open-label/unmasked design
- Low crossover, leaving the question of whether to include lurbinectiden in initial maintenance therapy or to save its use following disease progression on atezolizumab monotherapy
- Randomized and included only those without progression following induction therapy and an ECOG performance status of 0-1; preferentially selecting for healthier patients and limiting external validity
- Excluded those with brain metastasis
- Underrepresentation of some racial groups, including African Americans
Authors’ conclusions:
In conclusion, the addition of lurbinectedin to first-line atezolizumab maintenance therapy for ES-SCLC resulted in significant and clinically meaningful improvement in progression-free survival and overall survival. The safety profile of the combination was consistent with expectations and was considered manageable overall. These results highlight the potential of lurbinectedin plus atezolizumab as a promising novel treatment option in the first-line maintenance setting of ES-SCLC.
Cost (per Lexidrugs Online 2025):
- The cost of a single 4 mg vial of lurbinectedin is $9,900, and with an average BSA of 1.8 m2, the average person would require two vials, costing $19,800 per 21-day cycle
- 1200 mg/20 ml Atezolizumab vial is priced at $695.36 per ml, equating to a total cost of $13,907.20 per 21-day cycle
- These calculations do not include the cost of a G-CSF agent, which would be recommended with lurbinectedin therapy
Special Considerations/Populations:
- Although overall survival benefits were observed in the treatment group, certain subgroups, including patients with an ECOG score of 0, elevated LDH at randomization, or prior prophylactic cranial irradiation, did not demonstrate statistically significant benefit in the subgroup analysis. These subgroup analyses were exploratory, with wide confidence intervals and limited sample sizes, lacking power for interpretability
- The authors note that the efficacy outcomes are measured from the time of randomization into the maintenance phase of treatment, which is different from previous trials that included the duration of induction therapy, making it difficult to compare across studies
- At the time of analysis, 9% of the atezolizumab monotherapy group had received lurbinectedin as a follow-up treatment
- Measurable disease wasn’t required for maintenance randomisation eligibility, with about 26% not having measurable disease at maintenance randomization. ORR and duration of response were assessed only in those with measurable disease (non-randomized), and in the case of duration of response, only included those who achieved a confirmed objective response after randomization. 88% of patients had already achieved an objective response to induction therapy, so the observed ORRs of 19% and 10% represent additional responses beyond initial tumour burden. Given the limited number of patients in the ORR analysis, results should be interpreted cautiously
Final Thoughts
- Overall, this trial was well-designed and provides promising evidence of OS and PFS benefit for patients with extensive-stage SCLC, a historically aggressive disease with poor outcomes. Advancements in the first-line maintenance setting are particularly important, as high progression rates often translate directly to mortality, leaving many patients unable to access later lines of therapy.
*Information presented on RxTeach does not represent the opinion of any specific company, organization, or team other than the authors themselves. No patient-provider relationship is created.

