MANDALA Trial
The New England Journal of Medicine published the MANDALA trial online in May 2022. This post is going to be a journal club breaking down the MANDALA trial and what change it may bring to our clinical practice.

The New England Journal of Medicine published the MANDALA trial online in May 2022. This post is going to be a journal club breaking down the MANDALA trial and what change it may bring to our clinical practice.
If you would like to listen to the article instead of read, play the audio file below!
Background/Overview
Title: Albuterol-Budesonide Fixed-Dose Combination Rescue Inhaler for Asthma
Citation: Papi A, Chipps BE, Beasley R, et al. Albuterol-Budesonide Fixed Dose Combination Rescue Inhaler for Asthma. NEJM 2022 May;386(22):2071-2083. https://www.nejm.org/doi/10.1056/NEJMoa2203163
Study purpose: To evaluate the efficacy and safety of as-needed (PRN) use of albuterol-budesonide compared with PRN use of albuterol-alone in patients with moderate-to-severe asthma.
Background: Asthma is a disease that presents as repetitive airway obstruction from an inflammatory response to specific antigens/stress factors. Treatment for acute asthma exacerbations includes short-acting beta2-agonists (SABAs). SABAs provide short relief, but do not resolve the underlying airway inflammation. Solely relying on PRN SABAs leads to poor control and worsening disease over time. This can also lead to a greater risk for severe asthma exacerbations. Severe attacks carry a higher morbidity and mortality burden.
The Global Initiative for Asthma (GINA) have recently updated their recommendations for controller (maintenance) inhaler use. The recommendations are specifically for symptom-based (or rescue) use in patients with mild asthma (GINA Step 1 and 2) and daily inhaler use (GINA Step 2 to 5).
- Old recommendation: SABA monotherapy (most commonly albuterol, brand name ProAir or Ventolin).
- New recommendation in 2020 update: combination of inhaled corticosteroid (ICS)+SABA (most commonly budesonide-formoterol, brand name Symbicort).
*Note: Albuterol and formoterol are the preferred SABAs as they have the fastest onset of action.
The use of a SABA-ICS combination inhaler in patients with moderate-to-severe asthma has not been well-studied for rescue inhaler use. The MANDALA trial seeks to fill this gap in literature.
Funding sources: Astra-Zeneca and Avillion (manufacturers of first-in-class fixed-dose combination albuterol-budesonide inhaler).
Methods
Study design: Multinational, phase 3, double-blind, randomized, parallel-group, event-driven trial; 24 week duration; 295 sites included.
Patient population: Symptomatic patients with asthma ages 4 or older and had at least one severe asthma exacerbation in the previous 12 months.
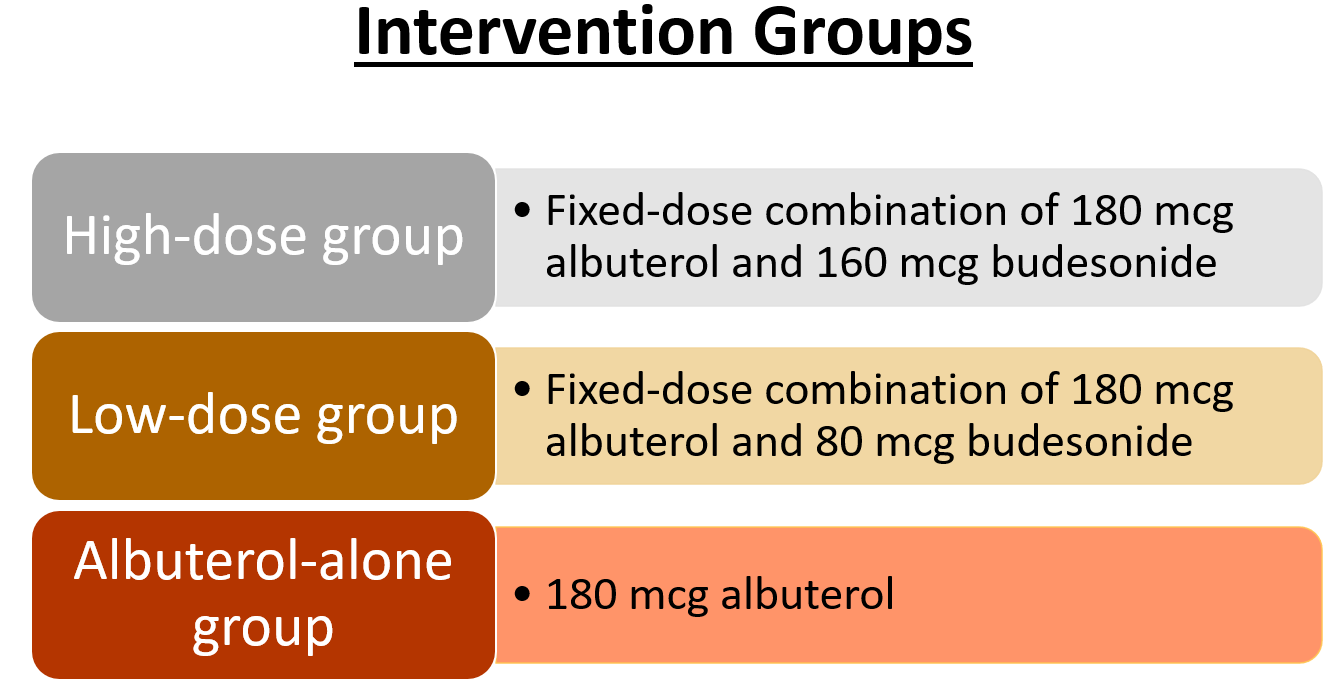
- Included patients were receiving a medium-to-high dose of ICS or a low-to-high dose of ICS-LABA (long-acting beta2-agonist) with or without another controller for at least 3 months with stable dosing for at least 4 weeks.
- Maintenance asthma medications were continued throughout the trial.
- Exclusion criteria: COPD, other lung disease, and use of systemic glucocorticoid or biologic treatments within 3 months of screening.
Outcome measures:
- Primary endpoints: first event of severe asthma exacerbation in a time-to-event analysis.
- Secondary efficacy endpoints: annualized rate of severe asthma exacerbations, total systemic glucocorticoid exposure for asthma during the treatment period, and response at week 24 on the appropriate validated Asthma Quality of Life Questionnaire (AQLQ) based on age.
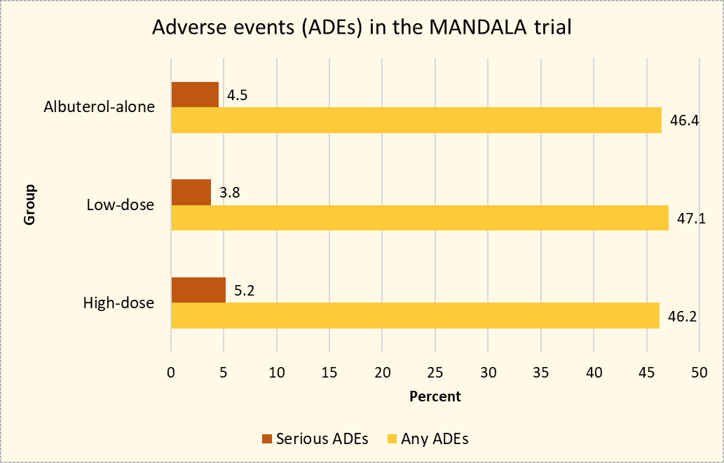
- Safety endpoints: incident adverse events and serious adverse events.
Statistical analysis: A preplanned efficacy analysis (termed "preplanned") and a prespecified intention-to-treat analysis (termed "prespecified") were completed. The prespecified analysis was to stay consistent with NEJM statistical guidelines and included all data, even when patients changed maintenance therapy during the study period. The preplanned analysis included only those patients who continued to receive their prescribed maintenance therapy.
- A sample size of 1000 adults/adolescents per group was estimated for 570 events which would provide a power of 87% to detect 25% lower risk of severe asthma exacerbation.
- Two-sided alpha of 5%.
- Probability of first severe exacerbation of 0.22.
- Time-to-event analysis performed with cox proportional-hazards regression model that adjusted for age group, geographic region, and number of severe asthma exacerbations in the 12 months prior to screening. Hazard ratios were reported.
Results
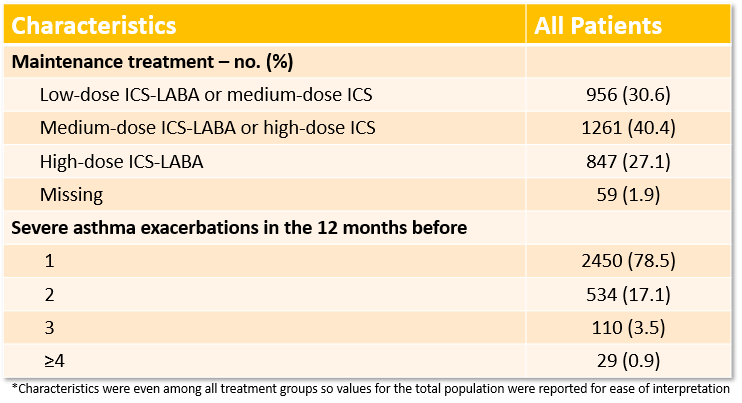
Baseline characteristics: A total of 3132 patients underwent randomization, and 3123 were assessed in the efficacy analyses. Of note, only 183 patients were less than 18 years old. The average age of all patients was 49 years old amongst the groups, 76% were 18-65 years old, 65% were female, and 81% identified as Caucasian. See below for characteristics on maintenance treatment and number of severe exacerbations in the 12 months prior to randomization.
Primary outcome results:
Both the prespecified and preplanned analyses showed a decreased risk over time in the combination groups compared to the albuterol-alone group.
Prespecified results (intention-to-treat):
- High-dose vs albuterol-alone: HR 0.74, 95% CI 0.62-0.89; p=0.001
- Low-dose vs albuterol-alone: HR 0.84, 95% CI 0.71-1.00; p=0.052
Preplanned results:
- High-dose vs albuterol-alone: HR 0.73, 95% CI 0.61-0.88
- Low-dose vs albuterol-alone: HR 0.83, 95% CI 0.70-0.99
Secondary outcome results:
Annualized rate of severe asthma exacerbations
- High-dose and albuterol-alone group rate ratio = 0.75 (95% CI 0.61-0.91)
- Low-dose and albuterol-alone group rate ratio = 0.81 (95% CI 0.66-0.98)
Total systemic glucocorticoid exposure for asthma during the treatment period (in prednisone equivalents)
- High-dose and albuterol-alone = 83.6 +/- 247.7 mg and 130.0 +/- 630.3 mg, respectively
- Low-dose and albuterol-alone = 94.7 +/- 318.2 mg and 127.6 +/- 619.8 mg, respectively
Response at week 24 on the appropriate validated AQLQ
- Significant response in the high-dose group compared to albuterol-alone in all age groups and in both the preplanned and prespecified analyses
- Non-significant response in low-dose group compared to albuterol-alone
Trial medication use:
- The use pattern of the PRN trial medications was similar in all three groups. The use was increased around the time of deterioration of asthma symptoms.
- On average, patients used 1.3, 1.3, and 1.4 doses of trial medication per day in the high-dose, low-dose, and albuterol-alone group, respectively.
Author's conclusions: The risk of severe asthma exacerbation was lower with PRN use of a fixed-dose combination inhaler of albuterol 180 mcg-budesonide 160 mcg compared to PRN use of an albuterol inhaler alone in patients with uncontrolled moderate-to-severe asthma.
Discussion
The primary endpoint was significant for the high-dose combination group compared to the albuterol-alone group in both the prespecified and preplanned analyses. The low-dose combination group fell short in the prespecified analysis, and cannot be declared as more effective at reducing the risk of severe asthma exacerbations compared to albuterol-alone. Secondary endpoints showed significant benefits for the high-dose combination, and adverse events were similar across the groups.
What this means is a potential change in practice for our patients with uncontrolled moderate-to-severe asthma who are receiving an ICS-containing maintenance therapy. Instead of an albuterol-only inhaler for PRN use (e.g. ProAir or Ventolin), the patients may now be better treated with a combination inhaler containing albuterol 180mcg-budesonide 160 mcg or equivalent SABA-ICS combinations and doses.
An important note: The use of trial medication was consistent among all three groups which adds validity to the results found. This is because there is not a potential bias of patients in one group using the rescue inhaler more frequently (or less frequently) than patients in another group.
Study strengths
- Preplanned and prespecified analyses: This gave the audience an understanding on if maintenance inhaler therapy would confound the results. Seeing as the primary endpoint in the high-dose group compared to the albuterol-alone group was significant in both analyses, the audience can conclude that maintenance therapy changes did not effect the results. This adds external validity to the study and more robust data that clinicians can use when applying this study to their clinical practice.
- Low-dropout rate: 93% of patients completed the initial 24 week treatment period. This increases external and internal validity of the results.
- Study design: The MANDALA trial was a multinational and double-blind study. In addition, it was randomized to control potential confounding variables. This increases internal and external validity as well.
Study limitations
- Lack of quantification of fraction of exhaled nitric oxide level: This would have allowed for direct assessment of anti-inflammatory effects.
- Small number of children: This limits external validity to this age of patients.
Final Thoughts and Clinical Application
Considering the last major update to the GINA guidelines took 14 years (2006 --> 2020), the results of the MANDALA are a big step for the uncontrolled moderate-to-severe asthma population. Reducing morbidity in chronic disease is always the goal!
Retail pharmacies will have an easier transition if combination SABA-ICS inhalers including albuterol-budesonide are brought to market and prescribed more frequently. The problems will arise when the new inhaler (manufactured by study funders AstraZeneca and Avillion) is not included on insurance formularies, hospital formularies, or has a high co-pay/out-of-pocket cost.
The results of the MANDALA trial evoke the question: what is the role of single-agent albuterol inhalers? The adult population with mild-to-moderate-to-severe asthma are most likely going to be on a SABA-ICS combination inhaler for PRN use moving forward. At this time, since children with moderate-to-severe asthma were not well represented in the MANDALA trial, this population will most likely continue to use albuterol-only inhalers for PRN use.
If SABA-ICS combinations do become the recommended rescue therapy for uncontrolled moderate-to-severe asthma patients, it will be important to counsel patients on the potential side effects of ICS inhalers and how to prevent them such as rinsing your mouth with water after each use and spitting it out to avoid oral candidiasis.
Extra credit question: Why are baseline characteristics important?
- The baseline characteristics allow us to understand what specific patient populations received benefit (or harm) from the intervention(s). As healthcare professionals, it is our job to interpret medical literature for use in the real world. For example, if a 75-year-old male patient of Asian ethnicity on a low-dose ICS-LABA maintenance inhaler with >4 severe asthma exacerbations in the last 12 months presented to your clinic, would you feel comfortable using the results of the MANDALA trial to prescribe a new SABA-ICS PRN inhaler? Most likely not, as the characteristics of this theoretical patient were not well-represented in the MANDALA study.

