Prodrugs: The Masters of Disguise
Call it a costume or hidden identity, some drugs have more to offer outside of their original form. A prodrug is an active drug chemically linked to a pro-moiety. We discuss the what, how, and why of prodrugs in this week's article.

Call it a costume or hidden identity, some drugs have more to offer outside of their original form. They term these masterminds "prodrugs." Their disguise is not as complicated as some Halloween costumes, however. Simply put, a prodrug is an active drug chemically linked to a pro-moiety which is cleaved off in the body. We discuss the what, how, and why of prodrugs in this week's article.
What are prodrugs
A prodrug, according to Merriam Webster dictionary, is a pharmacologically inactive substance (parent form) that is converted in the body (as by enzymatic action) into a pharmacologically active drug.
How exciting, right?
I mean, it gets the point across, but it can be difficult to digest the large terms and clinical relevance by that definition. In more common terms, a prodrug is a medication that does not have any effect in the body until it undergoes a chemical change into the active drug. The active drug can go and bind at receptors throughout the body and produce the desired therapeutic effect.

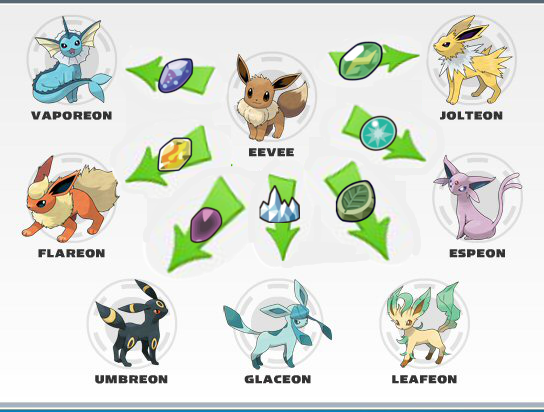
Think about it like the Pokémon, Eevee. By itself, Eevee is a normal-type, relatively unimpressive Pokémon to use in battle (sorry, basic Eevee lovers).
Once you hand Eevee a special item (fire stone, leaf stone, ice crystal, etc.), it transforms into a more specific, dangerous opponent in battle.
Eevee must undergo a transformation to become a more active Pokémon.
How do prodrugs work?
Unlike Eevee, prodrugs cannot simply find a special red blood cell or lymphocyte to hold on to and turn into the active drug. Prodrugs must undergo more complex chemical reactions in our bodies.
Prodrugs are maintained in their inactive form by a linked pro-moiety. This moiety is attached by a covalent linking or ionic bond.
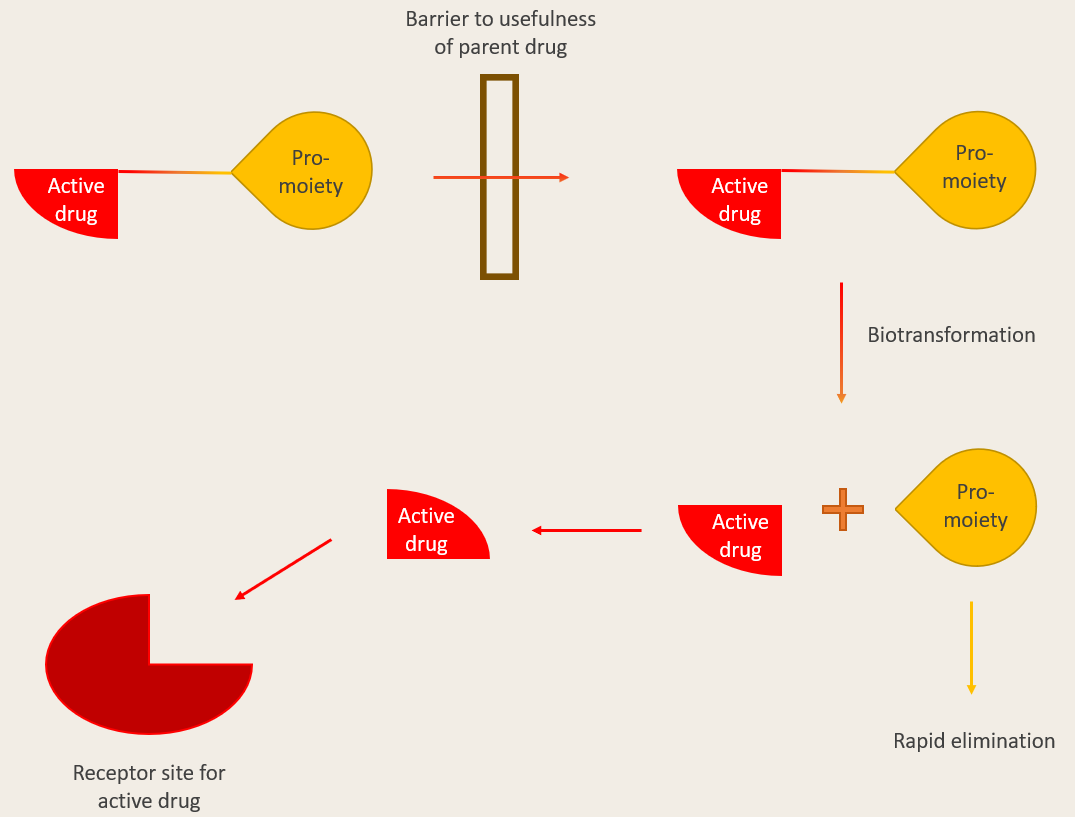
When a prodrug enters the body (i.e. the crossing the gut mucosa to enter systemic circulation), the pro-moiety is cleaved off. This happens through a variety of mechanisms:
- Hydrolytic digestive enzymes in the gut
- Carboxylesterases in the liver, duodenum, kidney, and brain
- Non-enzymatic chemical degradation (usually a more unstable parent compound and not as common on the market)
It is important that the prodrugs are non-toxic and rapidly eliminated from the body if not converted into the active drug.
Okay, now I know what they are,
but what specific drugs are prodrugs..?
Great question!
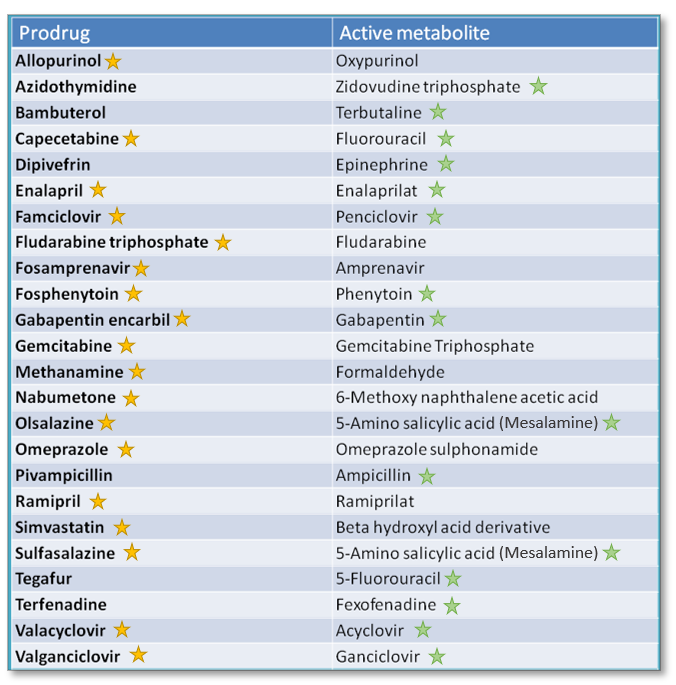
There are so many prodrugs on the market, and even more if you take a more liberal stance on the definition. Some authors use the term “prodrugs” to encompass active parent drugs which are also converted into active metabolites, such as benzodiazepines and tricyclic antidepressants.
Some common examples of active parent drugs with active metabolites (not included in the table below):
- Hydrocodone --> metabolized to hydromorphone
- Codeine (low affinity for mu receptors) --> metabolized to morphine (high affinity for mu receptors)
- Amitriptyline --> metabolized to nortriptyline
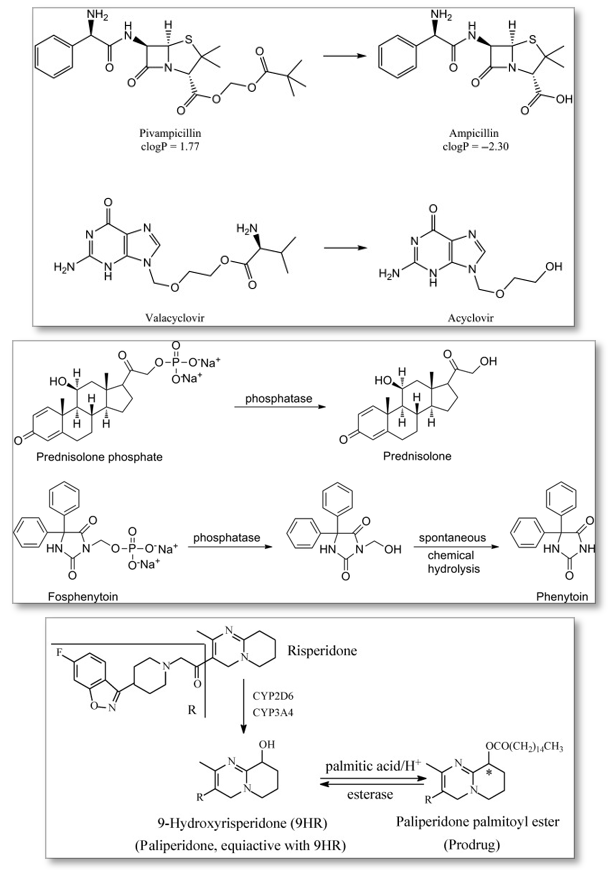
An ester bond is the most common form of prodrug linkage. Phosphate salts are the next most common. In Figure 1, Paliperidone palmitoyl ester conversion to 9-Hydroxyrisperiodone (9HR) is an example of an ester linkage. The conversion of prednisolone phosphate to prednisolone is an example of a phosphate salt.
Why are they important
A concept that was developed around 1958, prodrugs have been a pharmacologic breakthrough for numerous reasons:
- Bioavailability (poor aqueous solubility) – ex. Corticosteroids
- Poor absorption/permeability – ex. Ampicillin
- High first pass metabolism – ex. Propranolol
- Instability (short half-life) – ex. Dopamine
- Poor site specificity (the site of action of an active drug is rather nonspecific) – ex. Anticancer agents
- Incomplete absorption – ex. Epinephrine
- Pharmaceutical formulation difficulties (solubility with IV solutions)
- Adverse effects or toxicities
The pitfalls of prodrugs
Three main drawbacks come to mind: drug interactions, pharmacogenetics/age, and non-responders.
Drug interactions
- Many drugs affect the CYP450 enzymatic system. If these drugs inhibit the ability of the CYP enzymes to metabolize (biotransform) the prodrug, then the prodrug will remain in its inactive (parent) form and not have any therapeutic effect in the body. If the drugs induce (or increase) the ability of the enzymes to biotransform the prodrug, then the active drug will be more readily available in the body and potentially lead to toxic effects.
Pharmacogenetics/Age
- Newborns have a partially developed metabolic enzyme system and have difficulty metabolizing certain drugs. The same metabolic difficulty is seen in the older population because, as people age, enzymatic activity decreases. Children and young adults have the best functioning enzyme system for metabolizing drugs and can take full recommended doses. Consequently, newborns and older individuals frequently need smaller doses per pound of body weight than young or middle-aged adults.
- There are CYP450 mutations that can result in various polymorphisms. This can result in ultra-rapid, extensive, intermediate, and poor metabolizers. For example, if a patient is a poor metabolizer of CYP2C19, then they will not be able to convert the prodrug clopidogrel into its active form to produce antiplatelet effects. This will result in a non-responder (see below).
Non-responders
- One of the most common clinical scenarios I see in practice is non-responders to clopidogrel (Plavix). Clopidogrel is an antiplatelet medication given after a percutaneous intervention (PCI) or post-stroke (to give two examples, there are many more reasons to prescribe clopidogrel). Clopidogrel requires in vivo biotransformation to an active thiol metabolite. The active metabolite irreversibly blocks the P2Y12 component of ADP receptors on the platelet surface and results in decreased platelet aggregation (Lexicomp 2022). To assess clopidogrel activity, a P2Y12 assay can be run. If this assay comes back normal, that indicates that the patient is not metabolizing clopidogrel into its active metabolite and, consequently, not inhibiting P2Y12. If the P2Y12 assay is low, that means clopidogrel is appropriately being metabolized and inhibiting platelet aggregation!
- If a patient is a non-responder, especially to clopidogrel, a common action is to switch them to another P2Y12 inhibitor. Ticagrelor (Brilinta) is an option that does not require metabolism through CYP2C19.
Final thoughts/Clinical Application
I think the biggest takeaway from this article for clinical practice is staying diligent on drug interactions and knowledgeable on what prodrugs might be affected if prescribed. Our electronic health records are good at flagging drug-drug interactions. We can stay keen and interpret which drug-drug interactions are the most clinically meaningful.
Another tip if you are in doubt is to use the drug interaction checked on Lexicomp or Micromedex. You can also use these resources to identify which CYP enzyme a specific drug is a substrate for or if the drug inhibits/induces specific CYP enzymes.
- Lexicomp --> Lexi-drugs --> Specific drug monograph --> Interactions --> Metabolism/Transport Effects
- Micromedex --> Drugdex --> Specific drug monograph --> Pharmacokinetics --> Metabolism
Do you have some favorite prodrugs that were not mentioned in this article? Comment them down below!


