Spilling the Beans on Acute Kidney Injury
What does acute kidney injury mean when it comes to clinical practice? What does it mean for pharmacy medication management? This article will breakdown every definition and lab value to prepare you for managing AKI!
One of the first questions I was asked in residency was “what is the definition of acute kidney injury (AKI)?” I stared at my preceptor, speechless. I never thought about the textbook definition of AKI. A rough definition in my mind: a serum creatinine (SCr) that increased over a short period of time. Well, what "severity of increase” and what “period of time” would warrant a change in therapy in the clinical setting? We are going to cover this and more in this week’s clinical subtopic of nephrology!
Disclaimer: This post is meant to provide an overview of a clinical topic that may include (but is not limited to) information on pathophysiology, diagnosis, treatment, clinical pharmacology, medication management, adverse effects, and clinical pearls. References are included at the bottom of each post. This post is not to be used as medical advice for direct patient care, but as a guide for learning and discussion.
If you would like to listen to the article instead of read, play the audio file below!
Acute kidney injury (AKI) is a rapid decline in kidney function over a period of time. It is associated with an accumulation of waste products and volume; the “waste products” include medications. An accumulation of medication can lead to unwanted adverse effects, toxic concentrations, and further kidney damage. The organization KDIGO (Kidney Disease: Improving Global Outcomes) publishes guidelines for practitioners to use that range from chronic kidney disease (CKD) to renal transplant management.
There are multiple types of staging criteria for AKI. The RIFLE Classification (2004), AKIN Criteria (2007), and KDIGO Staging (2012) all use SCr as the basis of staging. There are other criteria based on urinary output that some experts use. All the staging criteria can be overwhelming. I have included a figure below outlining the KDIGO Staging criteria. I found this staging criteria easiest to learn and use in my clinical practice. There is also a chart below comparing all the staging criteria for your reference.
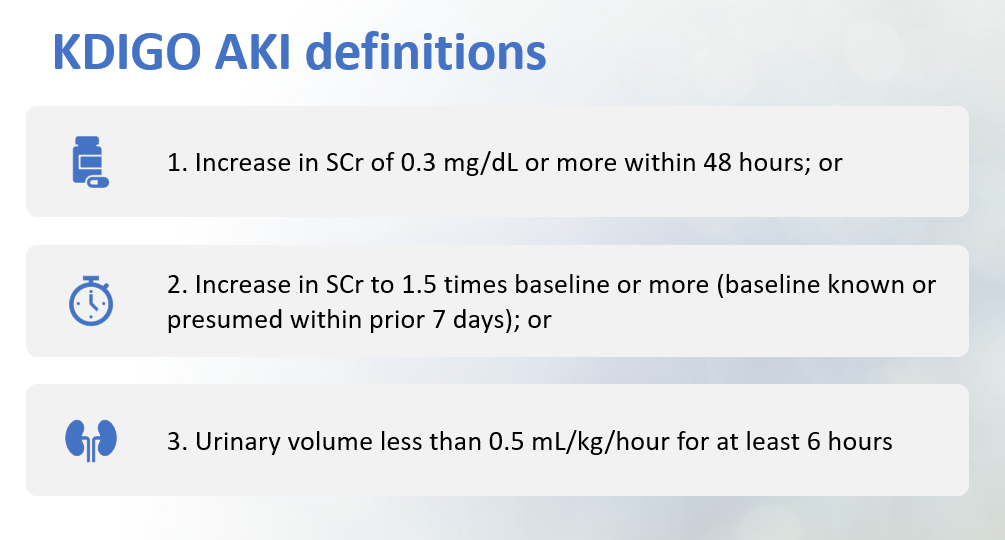
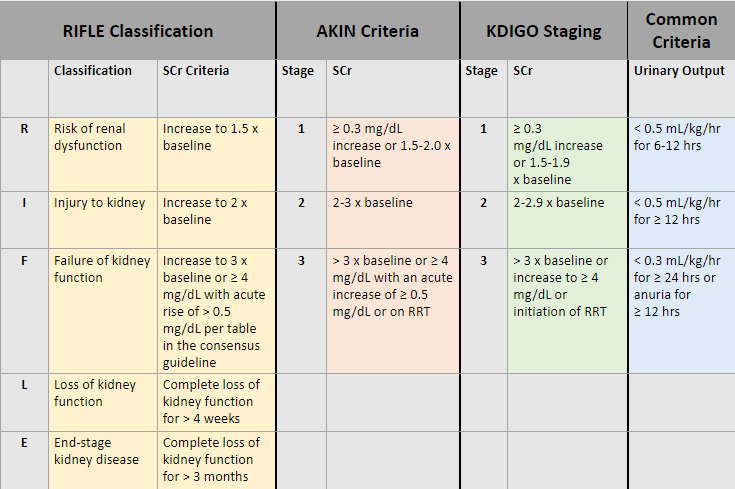
The use of various staging criteria for AKI addresses the use of the highly heterogeneous creatinine threshold and helps provide a more sensitive indicator of clinically relevant renal injury and absolute need (or not!) for dialysis.
Risk Factors
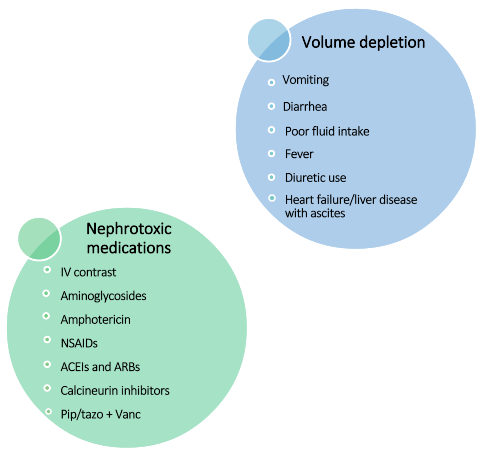
Patients are at greater risk for AKI when they have any of the following:
- Pre-existing CKD
- Volume depletion (see graphic below for examples)
- Use of nephrotoxic agents or medications (see graphic below for examples)
- Obstruction of the urinary tract
Classifications of AKI
There are four main classifications of AKI that you will want to familiarize yourself with in practice. Classifying AKI can help you understand how to best treat the AKI and how quickly the AKI is expected to resolve. Time to resolution of an AKI is important when dosing renally cleared antibiotics, for example. If a patient is suspected to have prerenal AKI from dehydration, the AKI itself should resolve within a short period of time (24-48 hours) after fluid resuscitation, and you can be more aggressive with antibiotic dosing.
Prerenal AKI
- Kidney is undamaged (initially)
- Cause: hypoperfusion of the kidney
- Physical signs: hypotension and volume depletion
- Urinalysis (UA) will be normal and concentrated (FeNa <1%)
Functional AKI
- Aka prerenal azotemia
- Kidney is undamaged
- Cause: reduced glomerular hydrostatic pressure
- Often medication related, but also occurs in patients with low blood flow and lack of compensation (heart failure, liver disease, older individuals)
- Concentrated urine
Intrinsic AKI
- Kidney is damaged
- Cause: acute tubular necrosis (ATN); less common includes acute interstitial nephritis, vasculitis, acute glomerulonephritis
--> think drug use, infections, or other identifiable insult
- Physical signs: normal blood pressure, usually euvolemic, check for signs of allergic reaction
- UA will have granular or epithelial cell casts and be unconcentrated
Postrenal AKI
- Kidney is undamaged (initially)
- Cause: bladder outlet obstruction is most common
- Physical signs: distended bladder, enlarged prostate
- UA may be nonspecific
- Imaging: radiograph or ultrasound may show hydronephrosis and aid in identification of obstruction
Treatment
Prerenal or Functional AKI
- Correct primary hemodynamics
- IV crystalloid fluids (normal saline, lactated ringer solution, etc.)
- Manage blood pressure for adequate perfusion to the kidneys if needed
- Hold medications that alter renal hemodynamics (ACEIs, ARBs, NSAIDs)
Intrinsic AKI
- No specific therapy; remove causative hemodynamic abnormality or toxin
- Avoid other nephrotoxic medications
- Manage fluid status, electrolytes, and nutrition
Postrenal AKI
- Monitor fluid balance
- Consult urology or radiology
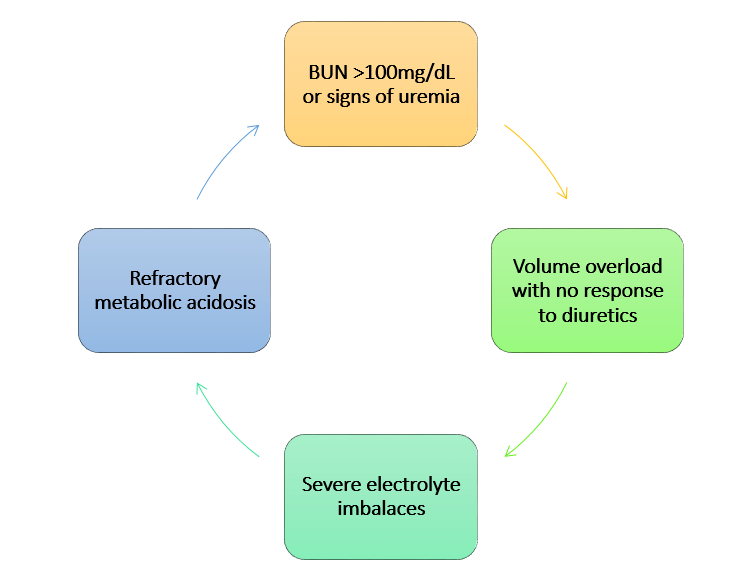
Indications for renal replacement therapy in AKI
What about FENa and FEU?
The fractional excretion of sodium (FENa) and the fractional excretion of urea (FEU) are two important values for determining the most likely type of AKI in a patient.
FENa (%) = [(urinary sodium)(serum sodium) / (urinary creatinine)(SCr)] x 100
FEU (%) = [(urinary urea)(SCr) / (urinary creatinine)(serum urea)] x 100
The FENa is the percentage of Na filtered at the glomerulus that is excreted in the urine. The FEU is the same except it is the percent of urea filtered and excreted in the urine.
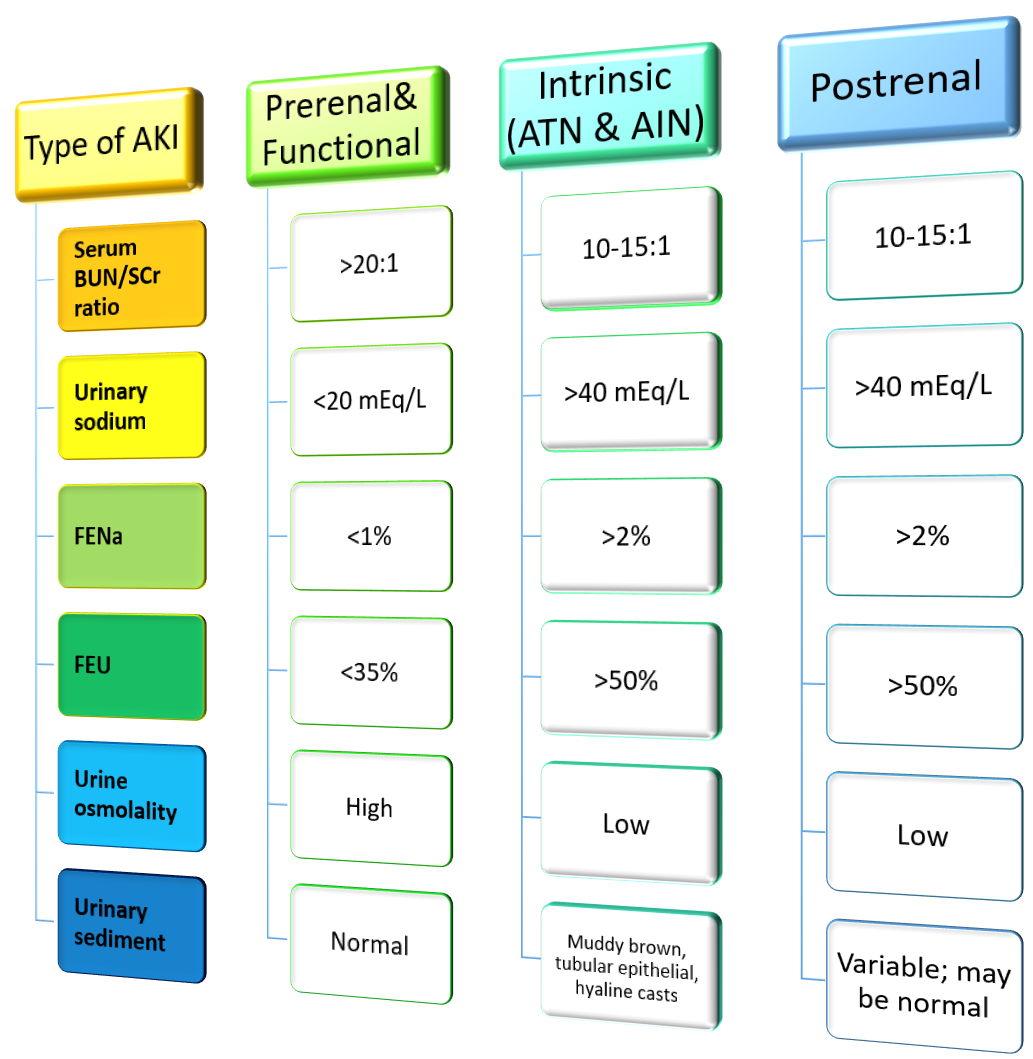
Be aware of loop diuretics
One thing to consider with FENa is if the patient is on loop diuretics. Loop diuretics will falsely elevate the FENa. This is because more sodium will be excreted in the urine. A patient with prerenal AKI on loop diuretics may present with a FENa >1%. The care team may think the patient does not have a prerenal AKI. This is something you can watch out for in practice!
A recently published study in heart failure patients found that FEU is also affected by diuretic use.
Clinical practice perspective from an inpatient hospital pharmacist:
In the hospital setting, we typically hold ACEIs, ARBs, NSAIDs, aldosterone antagonists, and diuretics unless clinically necessary. Often, I only see these medications initiated or restarted when the patient is near discharge. At my institution, we monitor SCr daily on all patients. We have an alert that fires when the patient has a significant increase or decrease in SCr. This allows us to streamline our work flow and be as efficient as possible.
Below is a screenshot of a patient who was admitted with AKI. After rehydration and holding nephrotoxic agents, you can see that their SCr dropped to 1.3 (close to their baseline of 1.0). An alert fired for me to review this patient's renally cleared medications and to make any necessary adjustments. My institution has protocols in place for certain medications allowing pharmacists to automatically adjust the dose.

On the other hand, if we have a patient on a scheduled vancomycin regimen, and an alert fires that their SCr increased from the day prior, we will switch the patient to a "dosing per level" frequency and collect a "random" vancomycin level to appropriately treat the patient and avoid toxic concentrations of vancomycin.
Watch out for IV contrast induced nephrotoxicity. Patient's often receive IV contrast for imaging in the hospital. Nephrotoxicity from contrast dye usually presents 2-5 days AFTER administration. The lab values in the screenshot below are from a patient who received contrast on 8/27 and developed AKI a few days later.

Do not underestimate the importance of renally adjusting medications! My rounding teams frequently ask what medications may be contributing to renal insult, and sometimes pharmacy can catch an AKI before the providers are aware. We are all valuable members on the healthcare team!
References
- Kidney Disease: Improving Global Outcomes (KDIGO). KDIGO Guidelines. Available at http://kdigo.org/home/guidelines/
- Billings FT 4th, Shaw AD. Clinical trial endpoints in acute kidney injury. Nephron Clin Pract. 2014;127(1-4):89-93. doi: 10.1159/000363725. Epub 2014 Sep 24. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4480222/
- Cox ZL, Sury K, Rao VS, et al. Effect of Loop Diuretics on the Fractional Excretion of Urea in Decompensated Heart Failure. J Card Fail. 2020 May;26(5):402-409. doi: 10.1016/j.cardfail.2020.01.019. Epub 2020 Jan 30. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7798124/

