The Andexxa Paradox
Andexxa withdrawn after ANNEXA-I showed higher stroke risk; clinicians return to 4F-PCC for DOAC reversal while new agents emerge.

There was a recent major shift in the clinical picture for reversing Factor Xa inhibitors, which include the commonly prescribed direct oral anticoagulants Eliquis (apixaban) and Xarelto (rivaroxaban). On December 22nd, 2025, Andexxa (andexanet alfa) was withdrawn from the market in the United States. After receiving post-marketing safety data from the ANNEXA-I trial, the FDA decided that the product’s risks outweighed its benefits. As a result, manufacturer AstraZeneca voluntarily withdrew the product’s Biologics License Application (BLA) and ceased sale and production of Andexxa in the U.S.1
What is Andexxa?
Andexxa was the first and only FDA-approved direct reversal agent for Factor Xa inhibitors. The available Factor Xa inhibitors on the market are:
- Rivaroxaban (Xarelto)
- Eliquis (apixaban)
- Edoxaban (Savaysa/Lixiana)
- Betrixaban (Bevyxxa)
Andexxa is a recombinant modified human factor Xa protein that acts as a decoy molecule. When administered, it binds to the anticoagulant molecules in the bloodstream, preventing them from inhibiting the patient's natural Factor Xa.
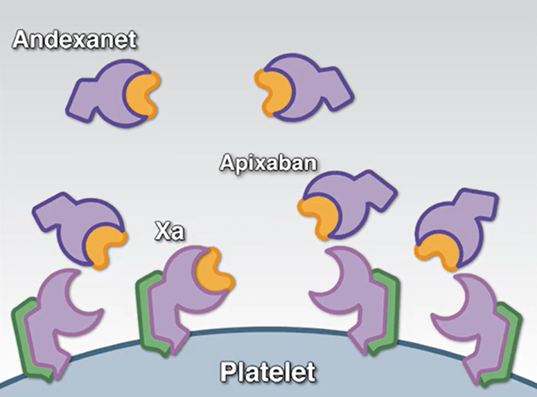
The drug received accelerated approval in 2018 based on its ability to lower anti-Factor Xa activity in healthy volunteers.2 Factor Xa activity was used as a surrogate marker that was expected to predict clinical success in bleeding patients. Under the FDA’s accelerated approval pathway, manufacturers must conduct confirmatory trials to prove that a drug’s lab-tested efficacy translates into real-world patient benefits. For Andexxa, this was the ANNEXA-I trial that eventually led to the product’s removal from the market.
The Data
The initial trial that formed the foundation for Andexxa’s accelerated approval was the ANNEXA-4 trial. This was a prospective, open-label, single-group study of 479 patients with major bleeding (primarily intracranial or gastrointestinal). Because there was no control group, every patient received andexanet alfa. There were three notable findings in this trial that led to the product’s approval:3
• 82% of patients achieved "excellent or good" hemostasis at 12 hours.
• Anti-Factor Xa activity was reduced by 92% for apixaban and 90% for rivaroxaban.
• Thrombotic events occurred in 10% of patients within 30 days.
The required confirmatory trial, ANNEXA-I, was a randomized controlled trial of 530 patients with acute intracerebral hemorrhage. They were randomized to receive either Andexxa or "usual care" (which was 4-factor PCC in approximately 85% of cases). The trial was stopped early in 2023 because Andexxa met its primary efficacy endpoint (≤35% volume increase in hematoma expansion, NIHSS score increase of <7, and no rescue therapy), but the final data was more complex:4
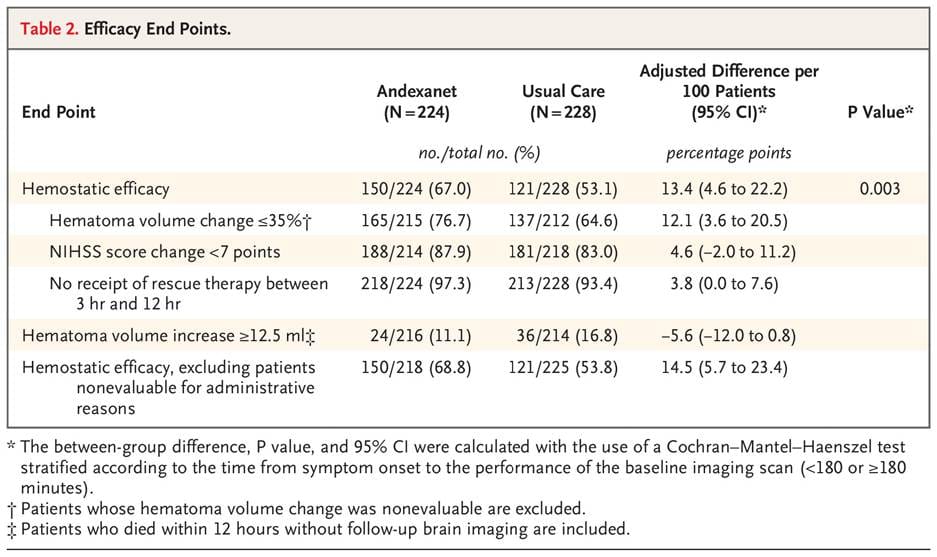
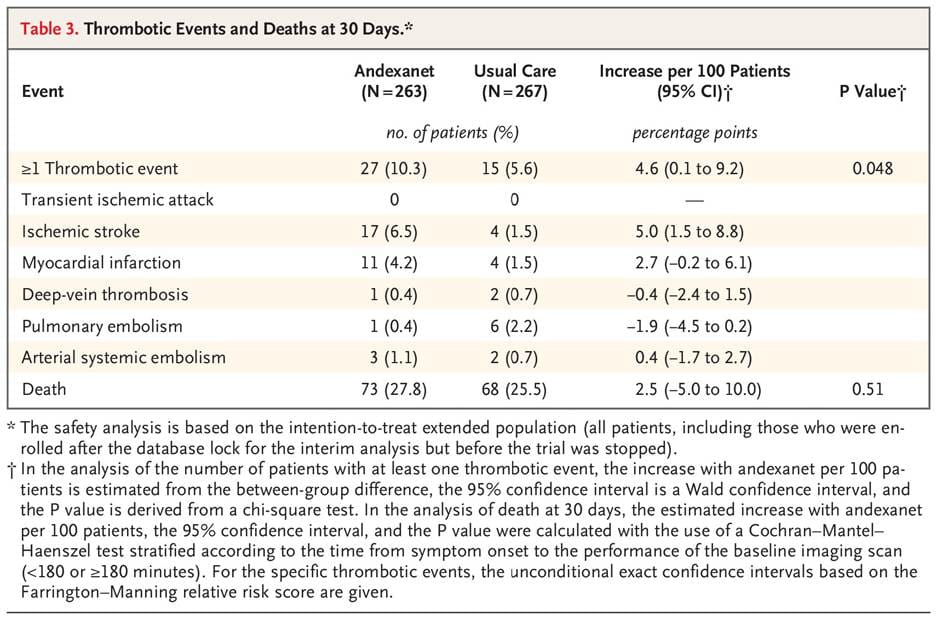
The ANNEXA-I results created a paradox. The drug did exactly what it was designed to do, but its pro-coagulant effect was so strong that it led to a nearly 4x increase in ischemic strokes compared to the standard of care. These troubling results are what led to the FDA deciding that Andexxa’s risks outweigh its benefits.
What Now?
Although the removal of Andexxa eliminates the only specific reversal agent for Factor Xa inhibitors, it is expected that the standard of care will once again be 4-Factor Prothrombin Complex Concentrate (4F-PCC), such as Kcentra. While Kcentra is FDA-approved only for the reversal of Vitamin K antagonists (like warfarin), it has been used as an off-label alternative intervention for apixaban and rivaroxaban reversal for years.5
Unlike Andexxa, which acts as a decoy to bind the drug directly, 4F-PCC follows a replacement strategy. It provides a concentrated bolus of the four Vitamin K-dependent clotting factors: II, VII, IX, and X, along with antithrombotic proteins C and S. By flooding the system with these factors, it aims to overwhelm the inhibitory effect of the DOAC. It does not remove the anticoagulant from the body; it instead provides the clotting factors necessary to form a clot despite the drug's presence in the body.
The Future
As we transition back to 4F-PCC, the focus remains on balancing hemostatic urgency with systemic safety. For now, the pragmatic replacement strategy of 4F-PCC provides a more stable safety profile. Moving forward, the clinical community is looking toward next-generation agents like ciraparantag, a small-molecule binder currently in Phase 3 trials that hopes to provide effective reversal without the heavy cost of thrombotic events.6
References
1: U.S. Food and Drug Administration. (2025, December 18). Update on the safety of Andexxa by AstraZeneca. https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/update-safety-andexxa
2: Endovascular Today. (2018, May 4). FDA approves Portola Pharmaceuticals’ Andexxa for the reversal of Factor Xa. https://evtoday.com/news/fda-approves-portola-pharmaceuticals-andexxa-for-the-reversal-of-factor-xa
3: Connolly, S. J., Crowther, M., Eikelboom, J. W., Gibson, C. M., Curnutte, J. T., Lawrence, J. H., Yue, P., Hoffman, M., Zotova, E., Videbaek, L., Abildgaard, U., & Milling, T. J., Jr. (2019). Full study report of andexanet alfa for bleeding associated with factor Xa inhibitors. New England Journal of Medicine, 380(14), 1326–1335. https://doi.org/10.1056/NEJMoa1814051
4: Connolly, S. J., Sharma, M., Cohen, A. T., Demchuk, A. M., Czlonkowska, A., Lindgren, A., Verma, A. K., Amarenco, P., Drouet, L., Peffault de Latour, R., & Milling, T. J., Jr. (2024). Andexanet for factor Xa inhibitor–associated acute intracerebral hemorrhage. New England Journal of Medicine, 390(19), 1745–1755. https://doi.org/10.1056/NEJMoa2313040
5: Tomaselli, G. F., Mahaffey, K. W., Cuker, A., Dobesh, P. P., Doherty, J. U., Eikelboom, J. W., Florido, R., Hucker, W., Mehran, R., Messé, S. R., Pollack, C. V., Jr., & Rodriguez, F. (2020). 2020 ACC expert consensus decision pathway on management of bleeding in patients on oral anticoagulants: A report of the American College of Cardiology Solution Set Oversight Committee. Journal of the American College of Cardiology, 76(5), 594–622. https://doi.org/10.1016/j.jacc.2020.04.053
6: Ansell, J. E., Bakhru, S. H., Laulicht, B. E., Steiner, S. S., Grosso, M. A., Brown, K., Dishy, V., Noveck, R. J., & Constant, J. (2021). Ciraparantag safely and completely reverses the anticoagulant effect of edoxaban in healthy adults. European Heart Journal, 43(10), 985–992. https://doi.org/10.1093/eurheartj/ehab558
*Information presented on RxTeach does not represent the opinion of any specific company, organization, or team other than the authors themselves. No patient-provider relationship is created.

